Highlights
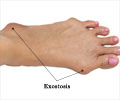
- Palovarotene can now suppress the formation of bony tumors in Multiple Hereditary Exostoses (MHE) patients
- Multiple osteochondromas is a hereditary genetic disorder in which many benign bone tumors grow at active areas of bone growth
- Multiple hereditary exostoses is a rare genetic condition that affects about 1 in 50,000 people worldwide
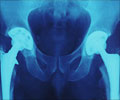
The condition is caused by mutations in two genes: EXT1 and EXT2. Individuals with these mutations develop painful, debilitating tumors, often repeatedly during their childhood and adolescence. Surgery and pain management are currently the only treatment options for MHE patients.
"Our study shows that palovarotene is a remarkably potent inhibitor of osteochondromas", says Yu Yamaguchi, M.D., Ph.D., a professor at SBP.
"In our mouse model of MHE, we were able to reduce bone tumors by more than 90 percent, which is a significant improvement over the previous drugs we have tested in the same mouse model." "Especially promising is that palovarotene has been tested for toxicity and side effects in humans and has been shown to be well tolerated," says Yamaguchi. "This means that timeline for getting the drug to the clinic for MHE may be shortened."
Clementia Pharmaceuticals licensed palovarotene from Roche Pharmaceuticals, which previously investigated the compound as a possible treatment for chronic pulmonary disease and evaluated its safety in more than 800 healthy volunteers and patients. Clementia Pharmaceuticals is planning to initiate a Phase 2/3 clinical trial in 2018 for patients with MHE.
"The long-awaited first clinical trial for a drug to treat MHE is now a reality. This breakthrough comes after years of working with medical professionals and scientists like Dr. Yamaguchi to achieve something we have all been desperately striving for, for many years."
- Toshihiro Inubushi, Isabelle Lemire, Fumitoshi Irie, Yu Yamaguchi. Palovarotene inhibits osteochondroma formation in a mouse model of multiple hereditary exostoses, Journal of Bone and Mineral Research (2017).DOI: 10.1002/jbmr.3341
Source-Eurekalert