- Study finds higher doses of the drug rifampin may lead to faster elimination of tuberculosis (TB) bacteria
- Rifampin is usually given along with three other drugs for around six months in TB therapy
- Findings could be beneficial and lead to a shorter treatment period for TB therapy
The findings are significant because with a high enough dose of daily rifampin a treatment period of less than the standard six months may be possible. The drug also has other benefits: it has the most potent sterilizing effect among the four first-line agents, is available throughout the world, and costs only pennies per capsule.
Earlier studies have looked at the effectiveness of killing the TB bacterium wit higher doses of rifampin given intermittently but found that the higher doses were more toxic than lower doses.
Treatment for tuberculosis consists of an intensive phase of 2 months, followed by a continuation phase of either 4 or 7 months (total of 6 to 9 months for treatment). The first-line anti-TB drugs given are isoniazid, rifampin, pyrazinamide, and ethambutol.
"Six months of treatment with four drugs--often delivered with support and supervision--represents a substantial burden on the health care system, as well as on the patient," Dr. Velásquez said. "Patients who cannot complete the full regimen may not be cured, which permits ongoing transmission and the development of drug-resistant TB."
Study design - Phase 2 trial conducted in Lima, Peru
Adults, 180 in number who were diagnosed with new, drug-susceptible TB participated in the trial.Participants were randomized equally into three groups.
During the first eight weeks of intensive therapy, the first group received a standard dose of 10 mg/kg/day of rifampin, the second received a higher dose of 15 mg/kg/day, and the third group received an even higher dose of 15 mg/kg/day. All three groups were given standard doses of the other 3 first-line anti-TB drugs along with rifampin during this phase.
During the following four months of continuation therapy, participants in all three trial arms received standard doses of rifampin and isoniazid.
Study results
- Each five mg/kg/day increase in rifampin increased the elimination rate of TB bacteria from sputum
- Higher rifampin concentrations also increased the elimination rates of TB bacteria from plasma
- The findings were valid even after adjusting for age, sex, and extent of disease
- The increased efficacy of higher doses did not result in more grade two or higher rifampin-related adverse events (liver toxicity and flu-like syndrome); in fact, the flu-like syndrome was not observed in this trial
"The difference was too modest at the tested doses for successful treatment shortening," Dr. Velásquez said. "However, these results, taken together with other recently published reports, support efforts to increase doses of rifampin to 35 mg/kg/day and possibly higher until the maximum tolerable dose is identified."
Tuberculosis
Tuberculosis (TB) is an infectious disease that mostly affects the lungs. The bacterium that causes the disease Mycobacterium tuberculosis can also damage other parts of the body.The mode of transmission of TB is primarily through the air; it spreads from one person to another when a person with TB of the lungs or throat coughs, sneezes, or talks. Symptoms include a bad cough that lasts 3 weeks or longer, weight loss, loss of appetite, weakness, and fever.
Not everyone who gets infected with TB bacteria becomes sick or has symptoms. As a result, there are two TB-related conditions: latent TB infection (LTBI) and TB disease.
If a person has TB infection, they cannot pass TB germs to other people but will need to stay on medicine for 3, 6, or 9 months, so they do not get TB disease. If a person has TB disease, they will need to take TB medications for at least 6 months; it takes at least 2 to 3 weeks of taking the pills to no longer spread TB germs to other people. Patients need to stay on the medicines to be cured even if they start to feel better.
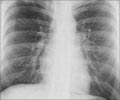
TB can be diagnosed through skin tests, blood tests, x-rays, and other tests.
Worldwide, there are nearly 10 million new cases of tuberculosis (TB) and 1.5 million deaths due to TB each year. TB treatment is currently recommended for six months. The preferred regimen for patients with newly diagnosed pulmonary TB consists of giving four drugs: isoniazid (INH), rifampin (RIF), ethambutol (EMB), and pyrazinamide (PZA). The drugs are given in two phases.
An initiation phase of 8 weeks where the medications are given 7 days/week for 56 doses or5 days/week for 40 doses, followed by a continuous phase of 18 weeks where the medications are given for 7 days/week for 126 doses or 5 days/week for 90 doses.
Shortening treatment by as little as two months would benefit patients and health systems.
References:
- Efficacy and safety of high-dose rifampin in pulmonary tuberculosis: a randomized controlled trial (http://www.thoracic.org/about/newsroom/press-releases/resources/rifampin-and-tb.pdf)
- Milstein M, Lecca L, Peloquin C, et al. “Evaluation of high-dose rifampin in patients with new, smear-positive tuberculosis (HIRIF): study protocol for a randomized controlled trial”. BMC Infectious Diseases. (2016) ;16(1):453. doi:10.1186/s12879-016-1790-x.
- Tuberculosis (https://medlineplus.gov/tuberculosis.html)
- Treatment for TB Disease (https://www.cdc.gov/tb/topic/treatment/tbdisease.htm)
- Tuberculosis Treatment (https://www.cdc.gov/tb/publications/pamphlets/TB_trtmnt.pdf)
Source-Medindia