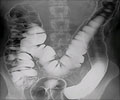
Cancer of the colon and rectum (colorectal cancer) is the second most common cause of fatal cancer. If identified early enough, this type of cancer is curable. Colorectal cancer (CRC) means cancer of the large intestine or rectum. The commonest symptoms are progressive constipation and stools mixed with blood. This cancer is best treated in its early stages. Treatment essentially consists of surgical resection of the colon followed by repair. If the cancer is very low in the rectum then after surgical removal, the patient has to live with a permanent colostomy in which the proximal colon is exteriorized to the left flank and stools are collected in a colostomy bag.
Although colonoscopy is a sensitive method to detect CRC, since it is time consuming and invasive, it is less used. More than half of the Americans aged 50 to 75 years do not comply with the recommended colorectal cancer (CRC) screening guidelines, which leaves 40 million individuals unscreened.A screening test for colorectal cancer (CRC) which is performed on blood would encourage more patients to undergo screening. If screening and early detection increases, expensive chemotherapies will be avoided more, resulting in cost saving to the healthcare system.
In a study conducted in United States plasma samples were collected from a number of patients with or without colorectal cancer. SEPT9 methylated DNA concentration was tested in these specimens.
The results showed that the improved SEPT9 methylated DNA test was more sensitive than other methods; the test had an overall sensitivity for CRC of 90% and specificity of 88%, detecting CRC in patients of all stages of the cancer. For early stage cancer (I and II), the test was 87% sensitive.
The SEPT9 test detected CRCs arising from all regions of the colon and rectum, including proximal tumors arising from the cecum and ascending colon.
In a small prospective study, the SEPT9 test detected 12% of adenomas with a false-positive rate of 3%. The adenoma detection rate was very low with this test; more than half of the subjects in the study with detectable SEPT9 were found to possess an adenoma or other polyp by colonoscopy.
Reference: Septin 9 methylated DNA is a sensitive and specific blood test for colorectal cancer; Jorja D Warren et al; BMC Medicine 2011.