- Boron can now be retained within cancer cells using a novel method
- Trapping boron within cancer cells long enough can kill them more effectively
- This could emerge as an effective anticancer therapy
The present study, published in Science Advances, focuses on how to keep the boron molecules within the cancer cells, without being exported out of the cells.
The study was led by Professor Nobuhiro Nishiyama, Laboratory for Chemistry and Life Science, Institute of Innovative Research, Tokyo Institute of Technology, Tokyo, Japan. He is also the Principal Research Scientist and Laboratory Head at the Innovation Center of NanoMedicine (iCONM), Kawasaki Institute of Industrial Promotion, Kawasaki City, Japan.
Background of the Study
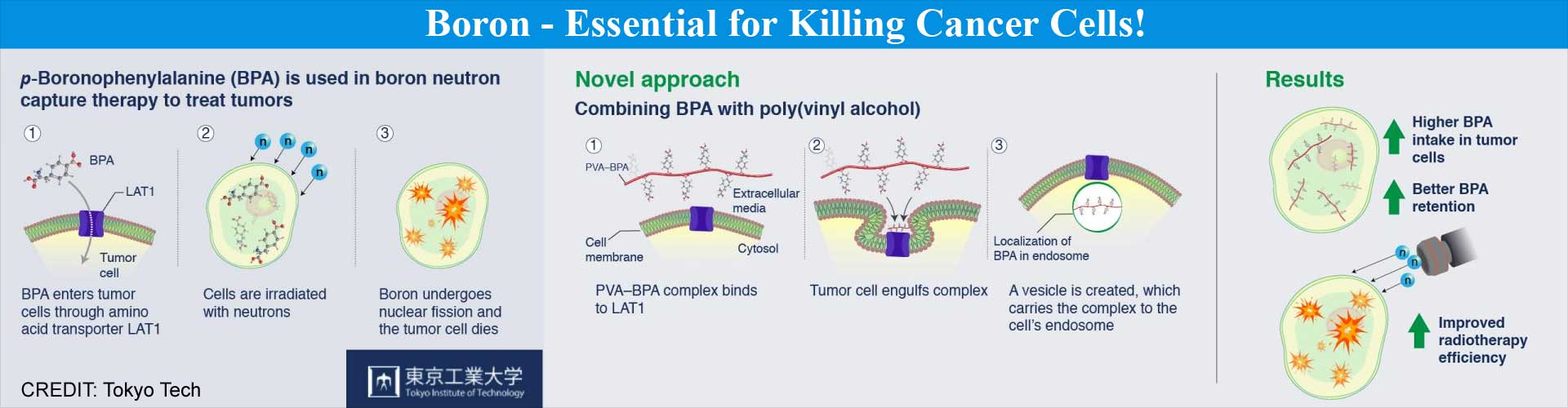
The uptake of boron into cancer cells occurs through a chemical compound known as p-boronophenylalanine (BPA), which has a boron molecule incorporated within the structure of the amino acid phenylalanine. The transport of BPA into the cancer cells is mediated by certain transporter proteins, which are unique to cancer cells.The most prevalent of these cell membrane transporter proteins is called Large neutral Amino acid Transporter 1 (LAT1), which transports specific types of amino acids, such as phenylalanine, across the cell membrane and into the cells. Therefore, LAT1 very efficiently transports BPA into the cancer cells, thereby enabling successful BNCT to be performed. Importantly, BPA is often regarded as the best available drug for performing BNCT.
Read More..
Drawback of BPA
Despite being a superior drug, BPA has a drawback. When the level of BPA increases within the cancer cells, it is transported out of the cells by an “antiport” mechanism, which is so-called because it transports molecules in the opposite (“anti”) direction i.e., out of the cells. As a result, BPA needs to be continuously infused into cancer patients for 30-60 minutes so that its level within the cancer cells remains adequate for performing BNCT. However, if there is any human error during the infusion process, the patient’s health could be jeopardized.How was the Drawback Overcome?
In order to overcome the drawback, the researchers explored other possibilities for retaining boron within the cancer cells for prolonged periods of time in order to successfully perform BNCT.Nishiyama indicates: “We assumed that modulating the presence of BPA inside the cell will ensure that it is not sent back out via the antiport mechanism.”
The researchers eventually found a way around the problem. They observed that when poly(vinyl alcohol) (PVA) was mixed with BPA, it formed a PVA-BPA complex that could be transported within the cancer cells.
Importantly, the structure of phenylalanine in BPA was not disrupted due to the formation of the PVA-BPA complex, thereby allowing LAT1 to recognize the molecular complex easily. However, as the PVA-BPA complex was too large to be transported through the narrow transmembrane channels, LAT1 facilitated the transport of the complex through the formation of minute structures called endosomes, which are produced by engulfment of the substance to be transported within the cell. These endosomes are formed by the invagination and pinching-off of the cell membrane during the process of engulfment and internalization of a trapped biomolecule, which in this case was the PVA-BPA complex.
The PVA-BPA complex remained protected within the endosome, thereby preventing it from being transported out of the cancer cells by the antiport mechanism. This ensured that the boron remained within the cancer cells long enough for killing them by BNCT. Importantly, this method was tested in animal models, which revealed that the anti-cancer effect of BNCT was significantly enhanced.
Concluding Remarks
The study clearly revealed that the addition of PVA is a simple and effective way to enhance the therapeutic potential of BPA.Nishiyama concludes:“This technique is effortless and offers a novel approach for drug delivery, focusing on the metabolic elimination processes of drugs. We will advance research on the PVA-BPA complex for clinical trials in cooperation with Stella Pharma Corporation, which has conducted BPA clinical trials.”
Funding Source
The study was funded by the Japan Science and Technology Agency, Japan Agency for Medical Research and Development, and Japan Society for the Promotion of Science.Reference:
- Poly(vinyl alcohol) Boosting Therapeutic Potential of p-boronophenylalanine in Neutron Capture Therapy by Modulating Metabolism - (https://advances.sciencemag.org/content/6/4/eaaz1722)
Source-Medindia