Highlights :
- USFDA has approved an implantable device called the Remede system to treat moderate to severe central sleep apnea
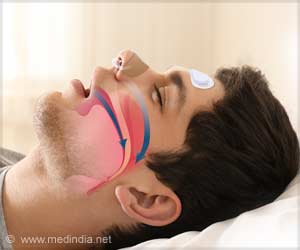
- In central sleep apnea, breathing stops momentarily due to failure of brain to stimulate the diaphragm
- Remedē system restores the lapse in brain signals to the diaphragm and ensures normal breathing again
“This implantable device offers patients another treatment option for central sleep apnea,” said Tina Kiang, Ph.D., acting director of the Division of Anesthesiology, General Hospital, Respiratory, Infection Control, and Dental Devices in the FDA’s Center for Devices and Radiological Health. “Patients should speak with their health care providers about the benefits and risks of this new treatment compared to other available treatments.”
Central Sleep Apnea
Sleep apnea occurs during sleep and causes a person to have one or more pauses in breathing or have shallow breaths.The pauses can be for a few seconds to minutes.In central sleep apnea, the brain fails to send signals to the diaphragm to breathe, thus leading to pauses in breathing during sleep for a period of 10 seconds or more before restarting again. This may lead to insomnia and may result in serious health issues, including an increased risk for high blood pressure, heart attack, heart failure, stroke, obesity, and diabetes according to the National Institute of Health’s National Center on Sleep Disorders Research. Existing common treatment options for moderate to severe sleep apnea include medication, positive airway pressure devices (for example, continuous positive airway pressure machine), or surgery.
Central sleep apnea is different from obstructive sleep apnea in which the patient attempts to breathe, but the upper airway is partially or completely blocked.
Testing of the Remede System in patients
The effectiveness of the Remedē System in reducing apnea hypopnea index (AHI), a measure of the frequency and severity of apnea episodes was assessed by the FDA.Results obtained from the data after six months from 141 patients was as follows
- With an active Remedē System implanted, AHI was reduced by 50 percent or more in 51 percent of patients .
- Without an active Remedē System implanted, AHI was reduced by 11 percent in patients.
Some of the precautions to be taken with the Remedē System is to not use it in patients with an active infection, those known to require magnetic resonance imaging and those with obstructive sleep apnea.
The FDA granted approval of Remede System to Respicardia Inc.
Reference:
- FDA approves implantable device to treat moderate to severe central sleep apnea - (https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm579506.htm)
Source-Medindia