A leading molecular diagnostic company has developed and commercialized epigenetic tests to improve the diagnosis and treatment of prostate cancer.
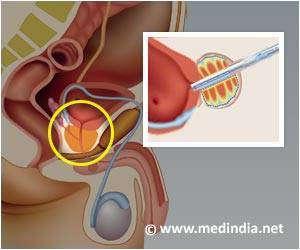
The data, presented at the European Association of Urology meeting in Glasgow, UK (October 9-11, 2014), confirmed that the epigenetic profile of genes generated by the test is solidly associated with established risk stratification metrics, such as Gleason Score (GS) and the NCCN (National Comprehensive Cancer Network) risk categories.In the presented study, hypermethylation levels of GSTP1, APC and RASSF1 were evaluated on biopsies from 102 men with various Gleason’s Grade (ranging from 3+3 to 4+4) and NCCN (ranging from very low to high) risk profiles. All men had gone through a 12-core prostate biopsy under routine clinical practice resulting in their prostate cancer (Pca) diagnosis. No epigenetic aberrations for any of these three genes were spotted in 20 control patients, who had non-cancer histology in all of their 12-core biopsy specimens.
For 46 men, the highest identified GS was 6, classifying these men as potential candidates for active surveillance; however, 17% (n=8) of them showed an epigenetic profile that is indicative of higher-grade disease.
Of the nine men with GS 4+3 or higher, 88% (n=8) had several epigenetic aberrations, in addition to 17 out of 27 (63%) of the men with GS 3+4.
When epigenetic profiling was compared to NCCN risk categories, similar outcomes were obtained, recognizing 79% (31 out of 39) of the men with intermediate or high NCCN risk profiles. Approximately one third of the patients in the very low and low risk categories showed similar epigenetic profiles.
In conclusion, epigenetic profiling using the genetic test on individual biopsy cores could help identify patients harboring aggressive prostate cancer, aiding patient management.
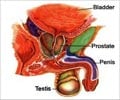
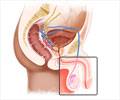
Prostate cancer is a malignant tumor in the prostate, a gland in the male reproductive system. Most prostate cancers are slow developing, but some grow relatively fast. These cancer cells can spread from the prostate to other parts of the body, particularly the bones and lymph nodes. They may initially cause no symptoms, but it can cause difficulty in urinating, blood in the urine in the later stage. Other late symptoms include feeling tired because of low levels of red blood cells.
This approach leaves men at high risk of occult cancer, leading to a high rate of repeat biopsies, often on cancer-free people. There is an urgent medical need for a clinically effective diagnostic test to address this issue. The current genetic test will help detect an epigenetic field effect or 'halo' linked with the cancerization process at the DNA level. This epigenetic‘halo’ around a cancer lesion can be present despite cells having a normal appearance under the microscope.
Which Company is Marketing the Test Kit
MDxHealth is a well-known molecular diagnostic company that develops and commercializes epigenetic tests to support cancer treatment. The company conducts tests based on proprietary gene methylation (epigenetics) technology and assist physicians with the diagnosis of cancer, prognosis of recurrence risk, and prediction of response to a specific therapy.
Source-Medindia





![Prostate Specific Antigen [PSA] Prostate Specific Antigen [PSA]](https://www.medindia.net/images/common/patientinfo/120_100/prostate-specific-antigen.jpg)





