- The United States Food and Drug Administration has approved the use of intranasal desmopressin for the treatment of nocturnal polyuria
- Desmopressin reduces urine production and therefore the number of times the person has to empty the bladder at night
- Other causes of nocturia should be ruled out before it is prescribed
- Increased intake of fluid before sleeping at night
- Overactive bladder
- Underlying illness like diabetes mellitus, diabetes insipidus or congestive cardiac failure
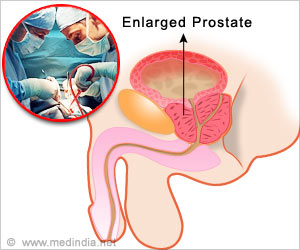
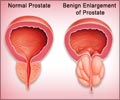
- Prostate problems like infection and prostate enlargement
- Urinary infection
- Swelling of the lower legs, due to re-distribution of fluid at night
- Pregnancy
“It is important to know that Noctiva is not approved for all causes of night-time urination, so patients should discuss their symptoms with their health care provider who can determine the underlying cause of the night-time urination and whether Noctiva is right for them.”
Studying the Efficacy of Desmopressin
The efficacy of desmopressin was studied in a two 12-week, randomized placebo controlled trial with 1,045 nocturia patients who were 50 years or older.Even though, these patients showed only a small reduction in the average number of night-time urinations with desmopressin when compared to placebo. The trial found that patients treated with the drug were able to halve their number of night-time urinations and were able to sleep for more nights with either one or fewer night-time urinations.
Desmopressin
Desmopressin is an analogue of the hormone vasopressin that is secreted by a small gland at the base of the brain called the pituitary. Vasopressin helps to reabsorb water from the kidneys and therefore reduces the amount of urine formed. It has been used to treat central diabetes insipidus, a condition characterized by reduced vasopressin levels.To understand if a person can use intranasal desmopressin, a 24-hour urine collection should be done. This will help to differentiate nocturia due to urine overproduction (nocturnal polyuria) from other causes of nocturia, where this drug cannot be used.
- People who drink excessive fluid
- Symptomatic congestive heart failure patients or uncontrolled hypertension due to its ability to cause fluid retention and worsen these conditions
- People at risk for low sodium levels. Desmopressin can cause low blood sodium levels that can be life-threatening especially in the elderly; blood sodium levels should be regularly monitored during treatment. Risk factors for low sodium levels include:
- Excessive fluid intake
- Presence of illnesses that can cause fluid or electrolyte imbalances
- Certain types of kidney damage
- Intake of loop diuretics or glucocorticoids
- People with nasal conditions like colds or allergies due to problems with absorption. It can be continued once the patient is better.
- During pregnancy or in children
- FDA approves first treatment for frequent urination at night due to overproduction of urine - (https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm544877.htm)