- Chronic plaque psoriasis is a chronic skin condition characterized by red scaly lesions that often requires lifelong treatment
- Current treatment options are not wholly satisfactory and also have unwanted side effects
- New targeted therapy using a biological agent is effective against psoriasis and exhibits minimal toxicity
Evolution of Psoriasis Treatment Over The Last Two Decades
Until the 1990s, treatment options were limited. In the last 15 years, research has identified inflammatory molecules called interleukins that are involved in the pathogenesis of psoriasis. Further research led to the development of antibodies or biologic therapy targeting or inhibiting these interleukins to treat psoriasis. Though these agents were effective in some patients, they were associated with severe toxicities. This was because interleukins involved in several other immune pathways were also disrupted leading to severe infections and immune system dysfunctions.Biological agents differ from traditional drugs in that they resemble molecules made by the body’s immune system naturally, and are adapted for therapeutic purposes.
How Tildrakizumab Therapy May Be Better, Compared To Current Psoriasis Therapy
Dr Kimball and her team through their research and observations have succeeded in identifying another interleukin specifically relevant to psoriasis. They developed a biological agent, tildrakizumab, an antibody that inhibits the specific interleukin involved in psoriasis. Trials have shown this drug to be effective in controlling symptoms of psoriasis with minimal side effects and could be a major milestone in the treatment of this debilitating condition.The current study team hopes their drug tildrakizumab would overcome the current gaps in psoriasis treatment and prove to be a game changer.
Testing Tildrakizumab In Humans – Details of the Study
The double-blind randomized controlled parallel studies, named reSURFACE 1 and 2, tested the efficacy of an antibody called tildrakizumab to control psoriasis in patients with moderate to severe disease.- Overall, more than 1,800 patients were included in trials conducted at 250 locations in the United Kingdom, the United States, Austria, Australia, Belgium, Canada, the Czech Republic, Denmark, France, Germany, Hungary, Israel, Italy, Japan, Netherlands, and Poland.
- Participants were randomly assigned to one of three groups:
- First group received 200 mg tildrakizumab
- Second group received 100 mg tildrakizumab
- Third group received an inactive placebo
- At the start of the study, all patients had a minimum of 30 percent of their body covered by psoriasis.
- At the end of the study, 12 weeks later, 65 percent of the participants demonstrated clear or almost clear skin, representing a 75 percent improvement. This was measured by the standard Psoriasis Area Severity Index (PASI).
- Less than 10 percent of the participants in the placebo group showed this level of improvement.
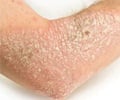
What Is Psoriasis?
Psoriasis is a chronic, sometimes lifelong, autoimmune skin condition, affecting both men and women. It is characterized by red itchy plaques and silvery scales, occurring anywhere in the body. The disease can be of varying severity, and is characterized by periods of remissions followed by flare-ups. Currently there is no cure though treatments keep the disease under check.Reference:
- Alexa B Kimball, MD et al. Tildrakizumab versus placebo or etanercept for chronic plaque psoriasis (reSURFACE 1 and reSURFACE 2): results from two randomised controlled, phase 3 trials. The Lancet, June 2017 DOI: 10.1016/S0140-6736(17)31279-5