Highlights:
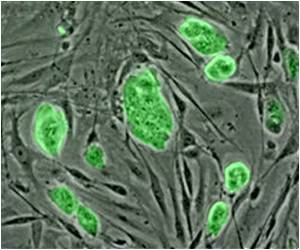
- After a heart attack the dead muscle of the heart is replaced by scar tissue - stem cell can help form new muscle from the scar tissue
- Heart muscle called cardiomyocytes are being produced from scar tissue (fibroblasts) using different techniques
- These vary genetically according to the technique used to produce them
Two approaches have been used to develop functional cardiomyocytes from fibroblasts, cells that normally produce fibrous or scar tissue.
- The fibroblasts are first converted into induced pluripotent stem cells (iPSCs), an immature form of cells, which then differentiate into cardiomyocytes. This process partially resembles the normal development of heart cells during embryonic development.
- The fibroblasts are directly reprogrammed into induced cardiomyocytes (iCM)
- Differences were observed in the expressed genes as well as a large number of noncoding RNAs in the cardiomyocytes produced by the different procedures.
- When the genes that were activated and not activated in the cells were compared, the iPSC cardiomyocytes (iPSC-CMs) were more similar to early embryonal cardiomyocytes with more active genes and a higher number of genes likely to be either activated or repressed. iCMs on the other hand, resembled adult cardiomyocytes more closely.
- The structure of the muscle cells was less organized and the contractility of the cells was less in the iPSC-CMs as compared to iCMs, reflecting the immaturity of the iPSC-CMs as compared to the iCMs.
- Longer culture times did improve the maturity of the iPSC-CMs but not as much as that of the iCMs.
- The cells differed in the genes responsible for metabolism. iPSC-CMs primarily expressed the glycolysis pathway genes, whereas iCMs primarily expressed the fatty acid oxidation genes.
Reference:
- Yang Zhou, Li Wang, Ziqing Liu, Sahar Alimohamadi, Chaoying Yin, Jiandong Liu, Li Qian3,'Correspondence information about the author Li Qian. Comparative Gene Expression Analyses Reveal Distinct Molecular Signatures between Differentially Reprogrammed Cardiomyocytes. Cell Reports. DOI: http://dx.doi.org/10.1016/j.celrep.2017.09.005
Source-Medindia