It also alleges that France's ANSM drugs and health products safety agency failed to provide proper warnings about the product's potential dangers.
The four victims, aged between 70 and 83, died at the end of last year and in the first three months of 2013 while taking the drug, the families' lawyer Philippe Courtois said.
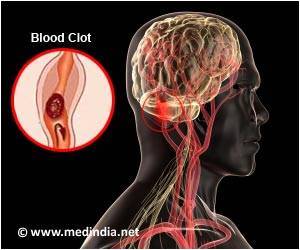
Pradaxa is a blood thinner prescribed to prevent strokes but it can cause serious internal bleeding and has been linked to a number of deaths.
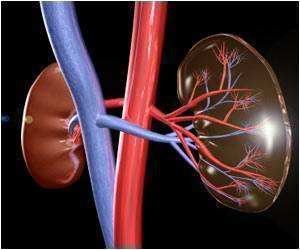
The complaint alleges that the potential side effects of the drug "were not sufficiently studied, especially in regards to the most fragile patients" such as the elderly and those suffering from kidney problems.
Courtois alleged that older people were under-represented in tests of the drug, "while they are the most exposed" to risks.
Advertisement
"Pradaxa is not a good product, but it could be a lot more dangerous to stop taking it without medical advice," said another lawyer for the families, Jean-Christophe Coubris.
Advertisement
The ANSM could not be reached for comment.
In a statement, Boehringer Ingelheim said it would "cooperate actively" with a French investigation.
A number of similar lawsuits against Pradaxa have also been filed in the United States.
Source-AFP