The letter was referred to PIP's saline implants, not the silicone implants that are at present the cause of concern, although both were manufactured at the same plant.
It is yet not established why the FDA warning failed to set off greater scrutiny of PIP's activities, or whether the US regulator shared its findings with the French authorities.
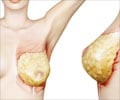
The French government is footing the bill for 30,000 women to have the Poly Implant Prothese (PIP) implants removed amid fears that they could rupture and leak a questionable type of silicone gel. In Britain, women have been recommended to visit their surgeon if they have any problem.
An international arrest warrant has been issued for Mas, who has not been tracked yet, but no charges have been filed so far.
Source-ANI