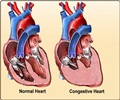
The FDA approved HUD designation of the 50cc Total Artificial Heart for destination therapy on January 15 and is available for patients who are at a risk of imminent death from non-reversible biventricular heart failure while its use in treating biventricular heart failure in pediatric patients was approved on January 30.
“The 50cc Total Artificial Heart is designed to fit patients of smaller stature, including many women and adolescents who are too small to receive the 70cc Total Artificial Heart. We are pleased that the FDA recognized the needs of these underserved patient populations and was swift in approving the HUD designations. Together, the 70cc and 50cc Total Artificial Hearts will fit almost all adult men and women, and many adolescents, including patients with congenital conditions”, SynCardia CEO and President, Michael Garippa said.
Source-Medindia