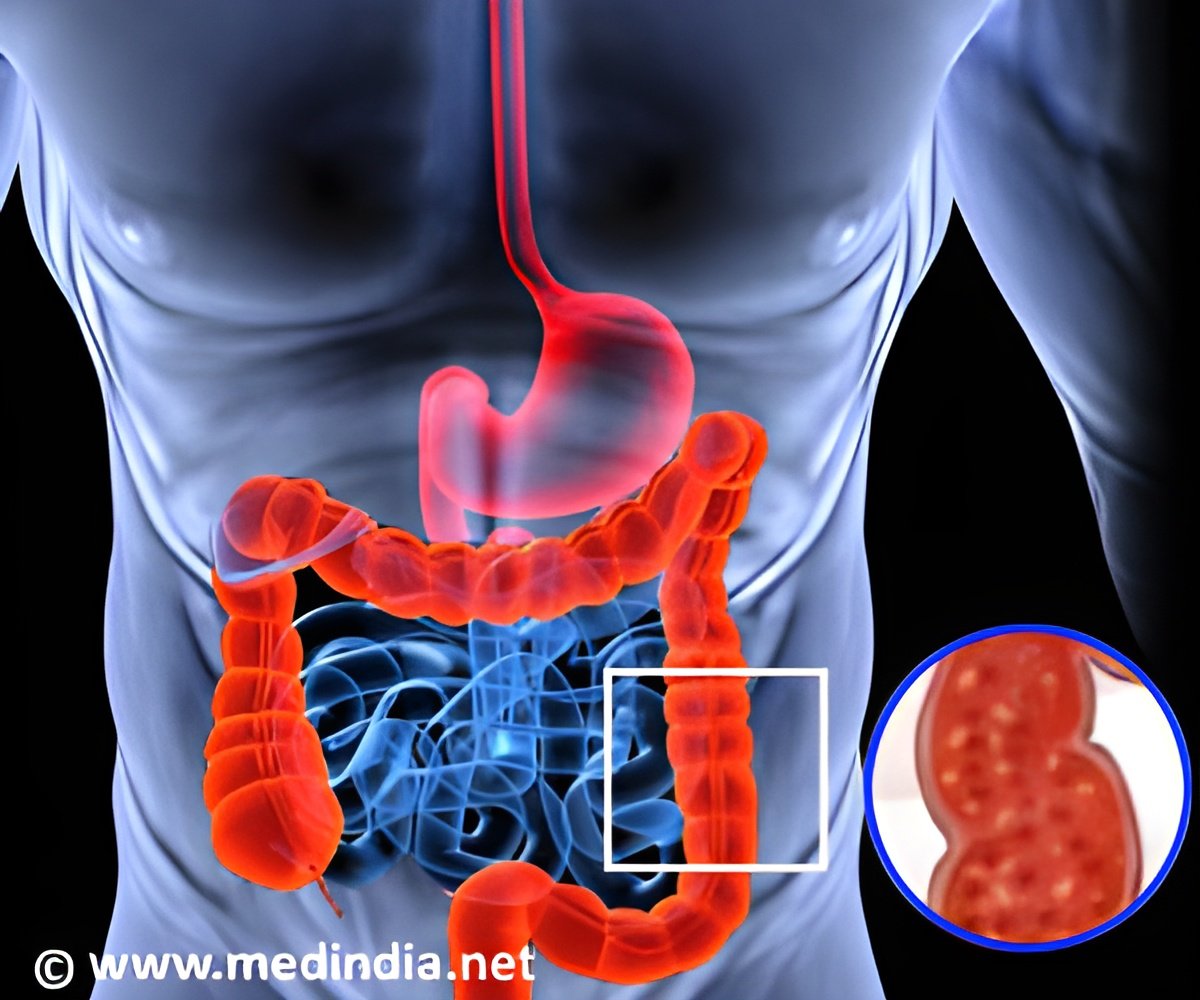
The US Food and Drug Administration announced that it has approved Indian pharmaceutical company Wockhardt’s generic treatment for ulcers to be marketed in the United States.
Wockhardt said that it has received the FDA approval to market the drug Lansoprazole in 15 mg and 30 mg strengths in the US. The company revealed that it already markets an over-the counter version of 15 mg Lansoprazole DR capsules and added that it will be launching the new products immediately.
Stating that the drugs will be manufactured in its Ankleshwar plant, Wockhardt released a statement saying, “Wockhardt will be manufacturing the Lansoprazole active pharmaceutical ingredient (API) in its facility at Ankleshwar, India and the delayed-release capsules of Lansoprazole at its facility in Aurangabad.”
Source-Medindia