GE Healthcare on Wednesday announced that it has received FDA approval for its mobile mammography screening device, Reuters reports.
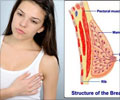
The device will make it easier to increase screening for breast cancer in rural areas, Reuters reports. According to a GE release, the Senographe Essential device will improve access to mammography screening worldwide.According to a study published in the June 15 issue of the journal Cancer, the proportion of U.S. women age 40 and older who said they have undergone a mammogram in the previous two years declined from 70% to 66% from 2000 to 2005.
"GE's goal is to enhance breast care for women worldwide and bring this technology to those who otherwise would not have access to it," David Caumartin, general manager of Global Mammography for GE Healthcare, said.
He added, "GE offers customers the broadest portfolio when it comes to breast imaging, and the new mobile Essential will be the top-of-the-line mobile product in the market featuring all the proven advantages of our Senographe platform".
Source-Kaiser Family Foundation
LIN/J