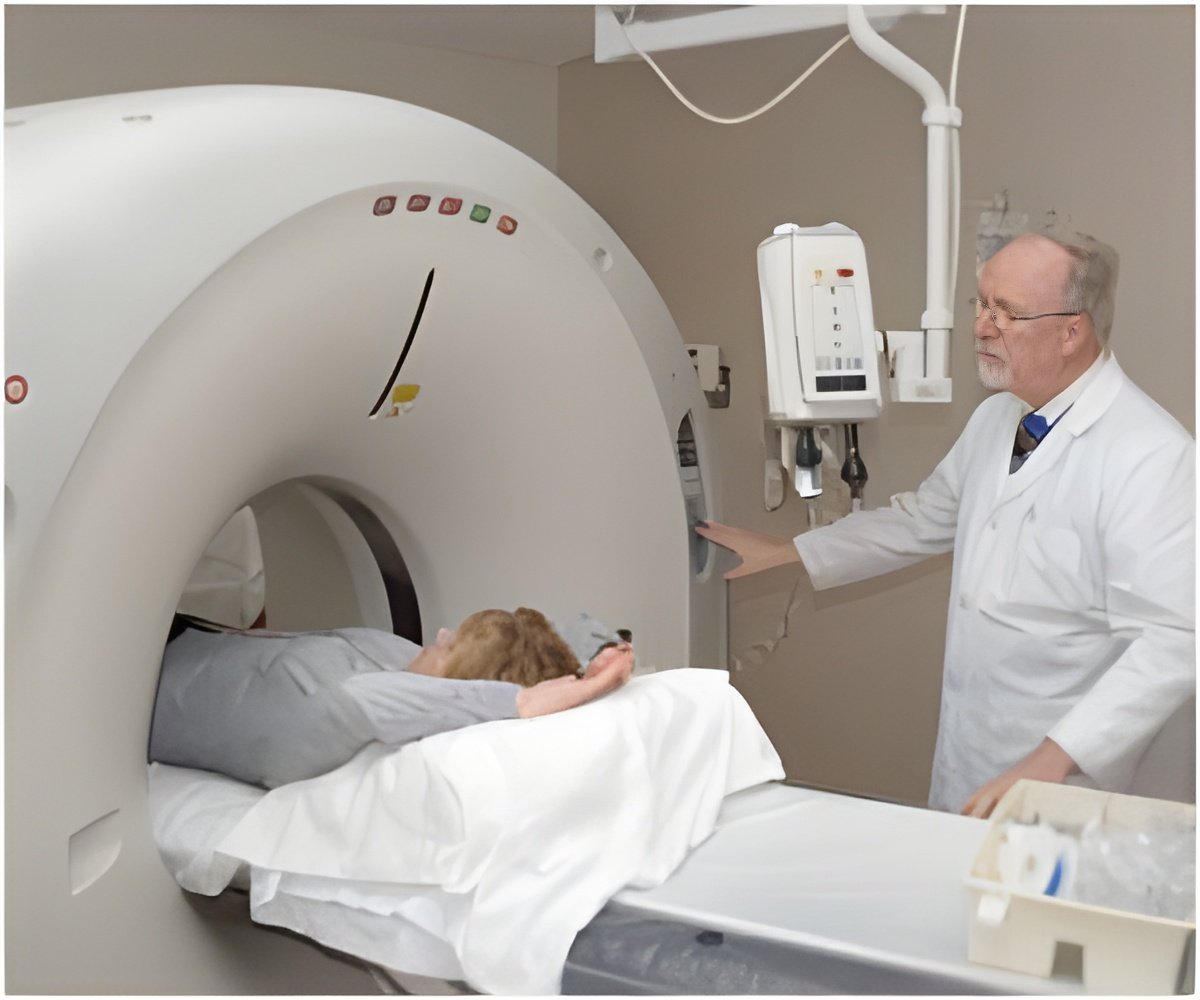
FDA approves ProMRI Eluna pacemaker, developed by BIOTRONIK, which allows patients with the device to receive full body MRI scans. This is the first available system that allows patients with single chamber pacing to receive scan of the heart. //
Cardiologists can monitor their patients and the implants with BIOTRONIK’s Home Monitoring system. The system is partially automatic and uploads unusual patient events and actions taken by the pacemaker to the company’s servers for physician review.
"We want every pacemaker patient to know that they can access MRI scans with our tested and approved systems,” said Paul Woodstock, BIOTRONIK Executive Vice President of Sales and Marketing. “BIOTRONIK’s latest device and ProMRI technology ensure that patients and their physicians will have the best diagnostic options available.”
Source-Medindia