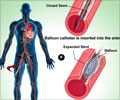
The SFA and proximal popliteal arteries present a challenging environment for stents.
The flexibility, radial strength and fracture resistance of the Innova Stent are designed specifically for this anatomy," said Richard Powell, M.D., section chief, Department of Vascular Surgery, The stent platform consists of a Nitinol self-expanding bare metal stent with an advanced delivery system.
It is also available in a range of sizes, including diameters from 5 mm to 8 mm and lengths of 20 mm to 200 mm.
"It features a hybrid cell architecture with open cells along the stent body and closed cells at each end of uniform and accurate deployment. This stent platform serves as the foundation for the new Eluvia Drug-Eluting Vascular Stent, designed specifically for the SFA.
The Innova Stent System was designed with an intuitive triaxial delivery system for precise, predictable stent placement and uniform deployment," said the company in a press release.
Advertisement
Advertisement