New Artificial Pancreas System Ilet Bionic Pancreas Gets Thumbs Up From FDA
Invented 20 years ago in the lab of Ed Damiano, a BU College of Engineering professor of biomedical engineering, the bionic pancreas combines an insulin infusion pump with algorithm-controlled dosing decision software (1✔ ✔Trusted SourceThe artificial pancreas: current status and future prospects in the management of diabetes
Go to source). He was inspired to develop the system by his son, who was diagnosed with type 1 diabetes when he was just 11 months old.
‘‘A device known as a bionic pancreas, which uses next-generation technology to automatically deliver insulin has been cleared by the US Food and Drug Administration (FDA).#diabetes #insulin delivery #artificial pancreas’’
Tweet it Now
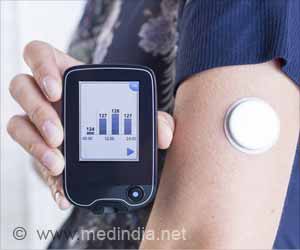
When paired with a Bluetooth-enabled glucose monitor, this device can deliver tailored insulin doses every five minutes, based on calculations of current and past glucose levels and the body’s reaction to past insulin deliveries.Small enough to be clipped on a bra strap or thrown in a pocket, the iLet means patients will no longer have to constantly measure their glucose levels and calculate, with help from their doctor, their correct insulin dose—a 24/7 endeavor. The iLet was cleared for people aged six years and older with type 1 diabetes.
Since FDA is committed to advancing new device innovation that can improve the health and quality of life for people living with chronic diseases, this approval action will provide the type 1 diabetes community with additional options and flexibilities for diabetes management and may help to broaden the reach of AID [automated insulin dosing] technology (2✔ ✔Trusted Source
Bionic Pancreas Outperforms Standard Care for Type 1 Diabetes in Trial
Go to source).
This milestone is particularly poignant to him as the news of FDA clearance coincided with the 24th birthday of my son, David, who developed type 1 diabetes as an infant, just over 23 years ago.
In fall 2023, Damiano and other researchers conducted a study and found that iLet helped adults and children maintain healthier blood glucose levels, outperforming existing standard-of-care methods—a significant step on its path to FDA clearance.
Advertisement
This is such a fantastic example of what a Boston University societal engineer does—advancing new ideas into real inventions driven for the betterment of others.
Advertisement
- The artificial pancreas: current status and future prospects in the management of diabetes - (https://nyaspubs.onlinelibrary.wiley.com/doi/10.1111/nyas.12431)
- Bionic Pancreas Outperforms Standard Care for Type 1 Diabetes in Trial - (https://jamanetwork.com/journals/jama/article-abstract/2798188)