"We don't have very effective treatments for this complication," said R. Loch Macdonald, M.D., study lead author and head of neurosurgery at St. Michael's Hospital, University of Toronto in Ontario, Canada. "The treatment in use now is very expensive and risky and doesn't work very well, and about half of the patients suffer permanent problems from it."
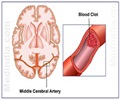
The Effect of Clazosentan on Clinical Outcome after Aneurysmal Subarachnoid Hemorrhage and Endovascular Coiling (CONSCIOUS-3) Study included 571 patients with a ruptured aneurysm treated with an endovascular coil, a device used to repair the ruptured blood vessel.
Researchers divided patients into three groups, each given either a placebo, 5 mg clazosentan or 15 mg clazosentan. They halted CONSCIOUS-3 prematurely after patients in CONSCIOUS-2 (a trial of patients whose ruptured aneurysm was secured by coiling), which ran simultaneously, showed no benefit from the drug.
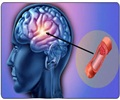
In CONSCIOUS-3, 27 percent of patients on placebo died, had vasospasm-related blood clots or neurological problems, or had rescue therapy to treat vasospasm, compared with 24 percent in the 5 mg/h and 15 percent in the 15 mg/h clazosentan groups. The odds of this combined endpoint were 53 percent lower at the higher clazosentan dose than with a placebo.
"Despite having less than half of the intended patients in the study, we were encouraged to see that at a high dose there was a significant reduction in vasospasm in patients initially treated with endovascular coiling," Macdonald said.
Advertisement
- Neurological deficits decreased with increasing clazosentan dose.
- Rescue therapy was three times less in patients with 15 mg/h clazosentan (7 percent) compared to those receiving placebo (21 percent).
- The occurrence of poor functional outcome or deaths within 12 weeks was not significantly different between placebo and medication groups.
- Patients receiving clazosentan had more lung complications, anemia and low blood pressure than those on placebo.
"The data suggests that the drug is effective at reducing delayed cerebral ischemia, its side effect profile is acceptable, and further studies with different doses, administration regimens, and the avoidance of other vasodilators may allow an improvement in outcome to be demonstrated," Macdonald said.
Advertisement