The hardest-hit state was Tennessee, where health officials said Friday the number of cases had risen to 29, including five deaths.
"I want to again express our sincere sympathy to the patients, family, and friends who've become victims of this tragic situation," state health commissioner John Dreyzehner said at a press conference.
Due to the long incubation period, health officials were urging patients who had received the injection as far back as July 1 to contact their doctors for testing.
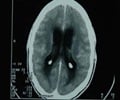
Fungal meningitis -- which inflames the protective membranes covering the brain and spinal cord -- is a rare infection which often goes undetected until it's too late because its flu-like symptoms can be mild at first.
Early detection and treatment -- which requires a hospital stay to administer intravenous anti-fungal medications -- can prevent the infection from causing permanent damage.
Advertisement
"The evidence indicates this is a product issue," he told reporters.
Advertisement
While further testing is required to confirm it was the source of the outbreak, the company has issued a recall of all of its products and shut down all operations.
Source-AFP