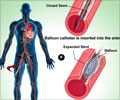
A set of treatments was compared to find the rate of thrombosis, adverse cardiac events or bleeding in patients. Bare metal stent (BMS) placement following elective percutaneous coronary intervention (PCI) such as stent placement and 1 month of DAPT was compared with 6 to 12 months for drug-eluting stents (DES).
Dean J. Kereiakes, the Christ Hospital Heart and Vascular Center, Cincinnati, and Laura Mauri, Harvard Clinical Research Institute and Brigham and Women’s Hospital, Boston, and colleagues randomly assigned 11,648 patients who received a bare metal stent or drug eluting stent. The patients were treated with aspirin and completed 12 months of dual antiplatelet therapy (DAPT) without bleeding or ischemic events. Further, they were prescribed thienopyridine or placebo at months 12 through 30.
Moderate or severe bleeding was less in the placebo but the rate of thrombosis and major cardiac events in the patients who continued thienopyridine was less than the placebo. Similarly, thrombosis rate and major cardiac events were fewer in BMS than DES while bleeding was more in BMS. The results comparing continued thienopyridine vs placebo in the cohort treated with DES demonstrated significant reductions in stent thrombosis and major cardiac events.
Source-Medindia