Significant flaws were seen in the evidence supporting the U.S. FDA recommendation of regular follow-up magnetic resonance imaging (MRI) scans for women with silicone breast implants.
The combined data suggested that MRI was fairly accurate in detecting implant-related problems. However, it was much more likely to detect problems in women with implant-related symptoms: 14 times more likely than in women without symptoms. Even in a mixed "screening sample"-consisting of some women with and some without symptoms-the detection rate was twice as high as in asymptomatic women.
Because most of the women in the studies had symptoms, the true accuracy of MRI for detecting implant-related problems in asymptomatic women was likely much lower. For ultrasound, reported accuracy rates varied widely.
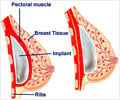
Silicone breasts implants have been extensively studied due to concerns about possible adverse health effects. Based on reports linking ruptured implants to autoimmune diseases, the FDA banned silicone implants in 1992. The ban was lifted in 2006, when repeated studies failed to confirm the association with autoimmune diseases.
However, the FDA recommended that women undergo regular MRI scans in the years after surgery to screen for implant rupture.
The researchers also note that in reported cases of implant rupture, the average age of the implants is over 10 years. Song and coauthors said , "[T]he benefits of screening within the first 10 years are unclear, and the effectiveness of such a screening program warrants further investigation."
Advertisement
The report has been published in the Plastic and Reconstructive Surgery.
Advertisement