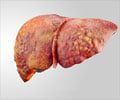
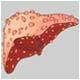
Dieters who use the weight loss and energy-boosting Hydroxycut supplements should immediately stop, the US drug regulator warned Friday after reports of liver injuries and one death.
The US Food and Drug Administration (FDA) said one person had died from liver failure. The agency has received 23 reports of liver problems linked with use of the products, ranging from jaundice to damage requiring liver transplant.In response, the FDA said, the maker of Hydroxycut -- Canada's Iovate Health Sciences Inc. -- had agreed to recall 14 products distributed by its US subsidiary.
"The FDA urges consumers to discontinue use of Hydroxycut products in order to avoid any undue risk. Adverse events are rare, but exist," said Linda Katz, interim chief medical officer of the FDA's Center for Food Safety and Applied Nutrition.
Hydroxycut products are marketed for weight loss, water loss, and as fat burners, energy-enhancers and low carbohydrate diet aids under the brand names Iovate and MuscleTech.
Hydroxycut Cleanse and Hoodia products were not included in the recall, the FDA said.
The health regulatory body said it had not yet determined which ingredients or dosages of the products were linked with the risks. It also stressed that liver injury related to Hydroxycut use was rare but patients who reported the ailment had taken the doses recommended on the bottle.
Advertisement
Source-AFP
LIN