In a US cancer centre, researchers are evaluating the safety and tolerability of a synthetic cannabinoid called dexanabinol (ETS2101).
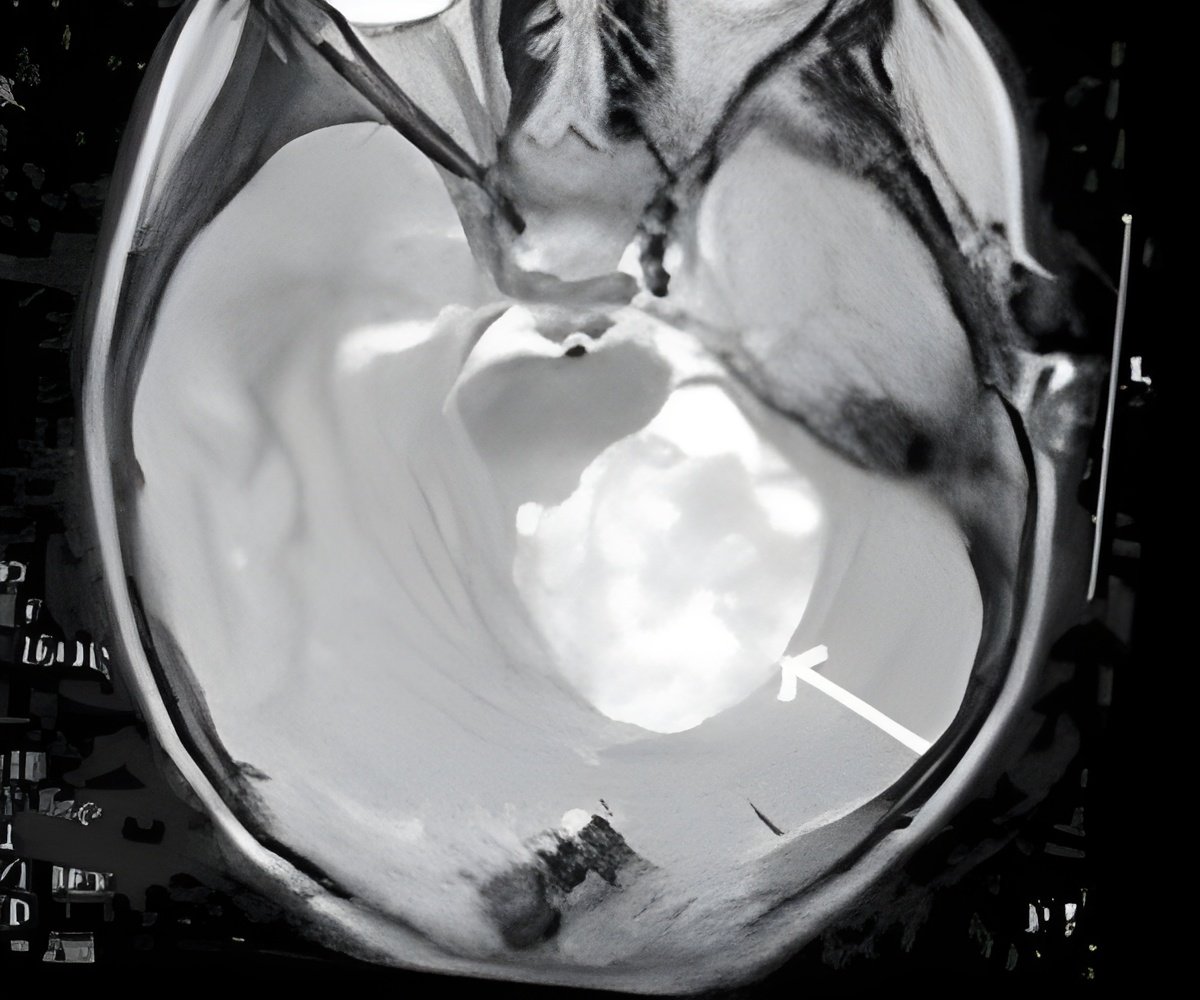
"In this Phase I study, we are examining the safety of multiple doses of dexanabinol, extent of penetration into the brain, and suitability for future trials," Santosh Kesari, MD, PhD, principal investigator, and director of neuro-oncology, UC San Diego Moores Cancer Center, said.
"What we hope to determine is the safe and optimal dose of drug in the brain," Kesari said.
Dexanabinol is a cannabinoid derivative that causes no psychotropic effects.
It was tested previously as a neuroprotective in patients with traumatic brain injury.
During these trials the drug was found to cross the blood-brain barrier.
Advertisement
Additional research in Kesari's lab demonstrated the drug's anti-cancer effects in patient-derived brain cancer cell lines.
Advertisement