Injections of denosumab could enhance the quality of life among patients suffering from osteoporosis caused by transfusion-dependent thalassemia.
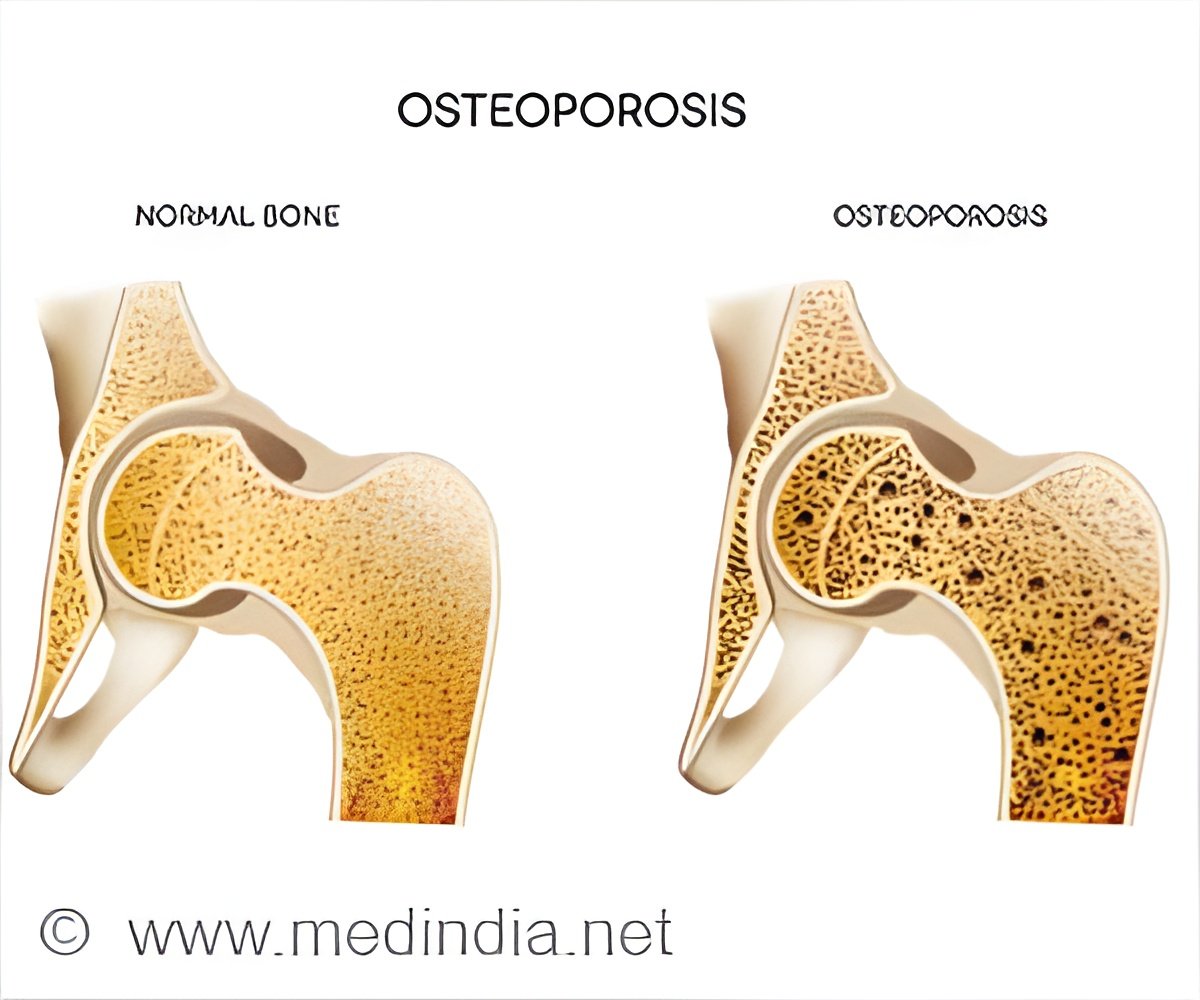
‘Patients suffering from osteoporosis as a result of other treatment options, should monitor their bone mineral density frequently to prevent further progression of disease.’
Read More..




People with osteoporosis have porous, weak bones that are susceptible to fractures and often cause pain. Current standard therapy for people with TDT and osteoporosis is to administer intravenous bisphosphonate agents such as pamidronate or zoledronic acid.Read More..
It is believed that people with thalassemia and osteoporosis exhibit elevated levels of the osteoporosis regulator gene known as RANKL. Denosumab, delivered under the skin instead of intravenously, is an FDA-approved anti-RANKL therapy that is not yet approved for use in people with TDT-induced osteoporosis.
Study methods: Researchers established a single-site, randomized, placebo-controlled, double blind phase IIb trial to evaluate the efficacy and safety of denosumab in patients with TDT and osteoporosis.
63 patients were randomly assigned to receive either 60mg of denosumab (n=32) or placebo (n=31) on days 0 and 180 over a period of 12 months. Each patient was also provided with daily supplements of calcium and vitamin D throughout the study.
Results: Each patient's bone mineral density was measured in the L1-L4 lumbar spine, the wrist, and the femoral neck. Patients receiving denosumab had a 5.92 percent increase in lumbar bone density compared to a 2.92 percent increase in the placebo arm.
Advertisement
"Not only is denosumab associated with improved bone health and reduced pain, but its ease of administration may very well make this drug superior to bisphosphonates for the treatment of osteoporosis in patients with TDT and osteoporosis," said senior study author Evangelos Terpos, MD, of the National and Kapodistrian University of Athens, Greece.
Advertisement
Conclusion: This study suggests that future studies should directly compare denosumab to bisphosphonates for the treatment of bone mineral density loss and pain management for people with TDT and osteoporosis.
### Additional Notes: This study was funded by Amgen.
Blood Advances. (http://www.bloodadvances.org), is a peer-reviewed, online only, open access journal of the American Society of Hematology (ASH) (http://www.hematology.org), the world's largest professional society concerned with the causes and treatment of blood disorders.
ASH's mission is to further the understanding, diagnosis, treatment, and prevention of disorders affecting blood, bone marrow, and the immunologic, hemostatic, and vascular systems by promoting research, clinical care, education, training, and advocacy in hematology.
Blood Advances® is a registered trademark of the American Society of Hematology
Source-Eurekalert