‘Gene editing approaches may prove clinically useful for the treatment of Duchenne muscular dystrophy (DMD).’
Tweet it Now
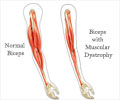
A gene therapy approved for DMD patients can restore dystrophin expression to about 1% of normal levels. CRISPR/Cas9 approaches have been shown to improve dystrophin expression in mice and human cells, but a critical step toward clinical translation of this method is a demonstration of efficacy in large animal models. Here, seeking to provide such evidence, Leonela Amosaii, Eric Olson and colleagues used viral vectors to deliver CRISPR gene editing components directly into the muscles of two one-month-old beagles with a naturally occurring genetic mutation representative of DMD. The animals' muscles were analyzed six weeks after treatment. Dystrophin expression was restored by up to 60% of normal levels in some muscle fibers, the authors say, and microscopic examination revealed an improvement in muscle integrity in the treated animals. In another experiment, the authors used the same viral vectors to deliver the gene editing components into the bloodstream of two beagles.
After eight weeks, the authors report, the animal receiving the highest dose showed a substantial boost in dystrophin expression: skeletal muscle had 25 to 70% of normal dystrophin levels and heart muscle had 92% of normal levels.
Preliminary analysis indicated that the treatment did not cause problematic immune system responses. Much work remains, however, before researchers could begin clinical evaluation of this approach in humans.
Source-Eurekalert