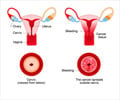
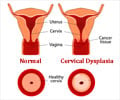
Drugmaker like GlaxoSmithKline India (GSK) and Merck face an anxious wait after the government decided to wait for results of full clinical trials before embarking on a cervical cancer vaccine drive in the country.
Drug Controller General of India Dr Surinder Singh said the results of the trials are awaited, “But, at the moment, we don’t see any urgency to include cervical cancer in the immunisation programme,” he added.Alarm bells were raised last fortnight when a school girl in the UK died after receiving the vaccine. However it later emerged that her death was not related to the jab.
The Indian Council of Medical research (ICMR) along with Merck is currently conducting a trial to assess the safety of the vaccine.
Source-Medindia
RAS