Psoriatic arthritis is a chronic inflammatory disease of the skin and joints and affects as many as 30% of people who suffer from psoriasis.
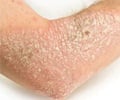
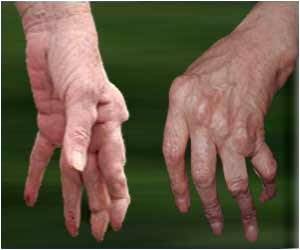
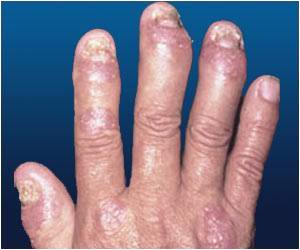
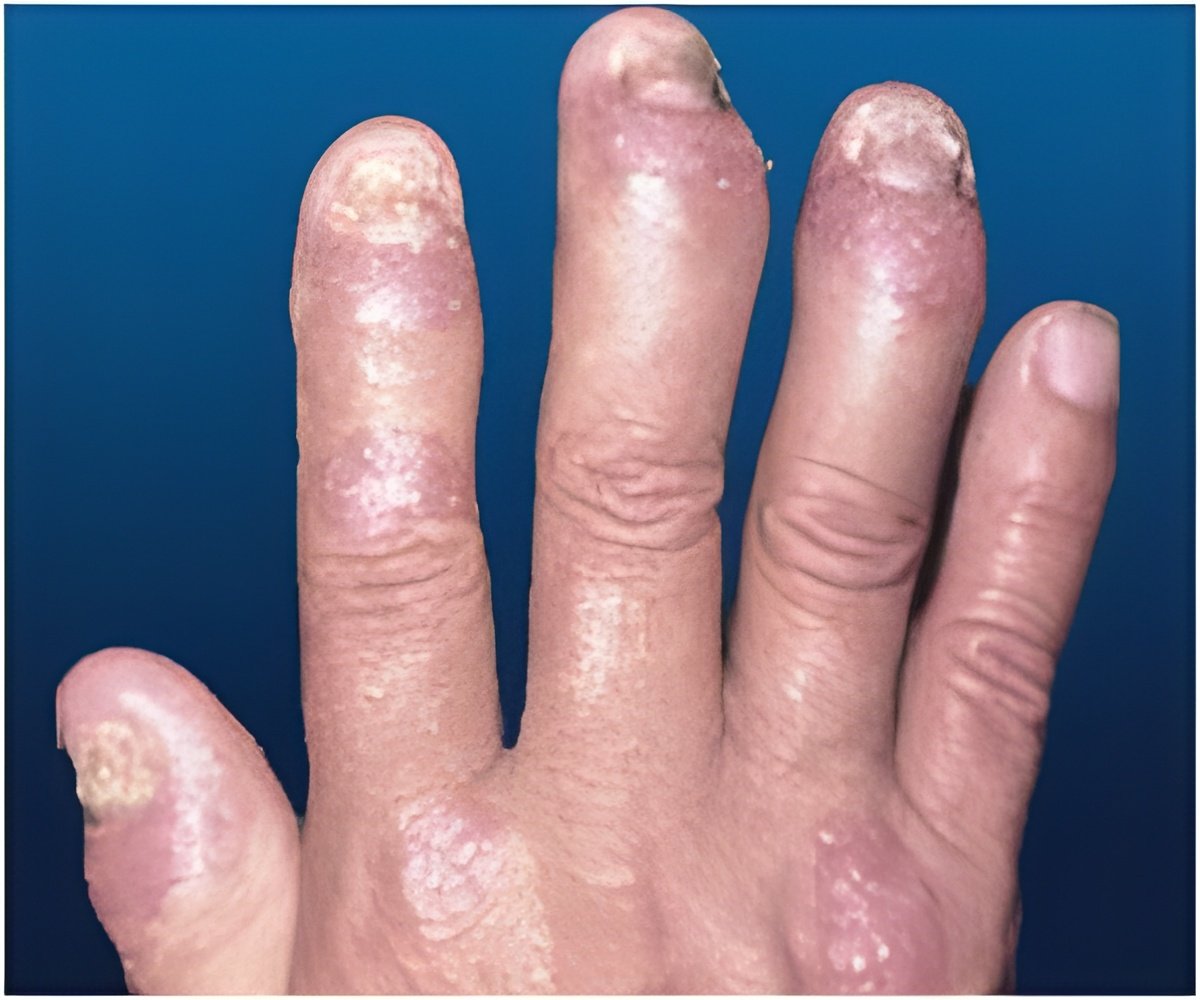
Psoriasis causes raised, scaly red and white patches on the skin. Psoriasis often affects the tips of elbows and knees, the scalp, the navel, the ears and around the genital areas or anus. In the case of psoriatic arthritis, the joints are affected as well, resulting in inflammation. Prolonged inflammation can result in joint damage, according to the American College of Rheumatology (ACR).
As in psoriasis, symptoms in psoriatic arthritis also come and go and vary from person to person. They even change locations over a period of time.
Brodalumab falls under a category of drugs called IL-17 inhibitors. IL-17 inhibitors block a signaling pathway that plays a vital role in triggering and developing inflammatory diseases.
A 68-patient trial of the biotech drug, brodalumab, was conducted and a 12-week data presented by Amben and its partner AstraZeneca Plc. The data showed the relative superiority of the drug over a placebo in terms of efficacy.
Advertisement
Findings at the end of 52 weeks showed 71 percent of patients who from the start received the 140 mg dose, 56 percent of patients who received 280 mg from the beginning and 50 percent of the original placebo group had hit ACR20.
Advertisement
ACR20 is a unit of measurement that signifies at least 20 percent improvement in signs and symptoms of psoriatic arthritis.
ACR50 indicates a 50 percent progress in disease signs and symptoms. This goal was reached by 47 percent of the original 140-mg patients and 27 percent of the original 280-mg patients at a year.
ACR70 was achieved by 22 percent of the first group and 7 percent of the second group.
Of the placebo group, those who switched to 280 mg, the ACR50 rate was 38 percent and ACR70, 14 percent.
Research firm ISI Group has predicted annual peak sales of about $2 billion for the drug.
Patients involved in the study suffered from psoriasis in varied degrees, and they had failed at least one therapy. About one half of them had stopped responding to injectable biologic medicines known as anti-TNF drugs, including Humira and Enbrel as the efficacy of anti-TNF drugs tends to diminish over a period of time.
"It’s hopeful that this (anti-IL-17) mechanism is central enough that they’re going to have responses despite the failure of anti-TNF therapy," Mease said.
Four cases of serious adverse events were reported, that included one patient who was severely infected and another one with complaint of abdominal pain, but no deaths were reported.
Mease, the study’s lead researcher, also noted that psoriatic arthritis is a genetic disorder that distinguishes it from other types of arthritis.
"There are also certain genes that are present in people who develop the arthritis that are not present in people with psoriasis. So there seems to be a heavy genetic component for determining who gets psoriasis and goes on to get psoriatic arthritis," he said.
At present, psoriatic arthritis is treated based on the degree of pain experienced by the patient. The treatment begins with painkillers, such as ibuprofen or naproxen..
Mease said methotrexate is given to treat patients suffering from both arthritis and psoriasis. Other drugs included in the biologic therapy that are helpful in treating both conditions are adalimumab, etanercept, golimumab and infliximab.
Findings of the study were published June 12 in the New England Journal of Medicine. They will be presented at the European Congress of Rheumatology’s annual meeting in Paris on Thursday.
Source-Medindia