‘New guidelines recommend the APBI technique for a broader group of patients by allowing younger patients to be eligible as well as those with ductal carcinoma in situ.’
Tweet it Now
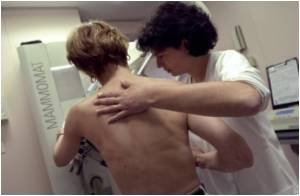
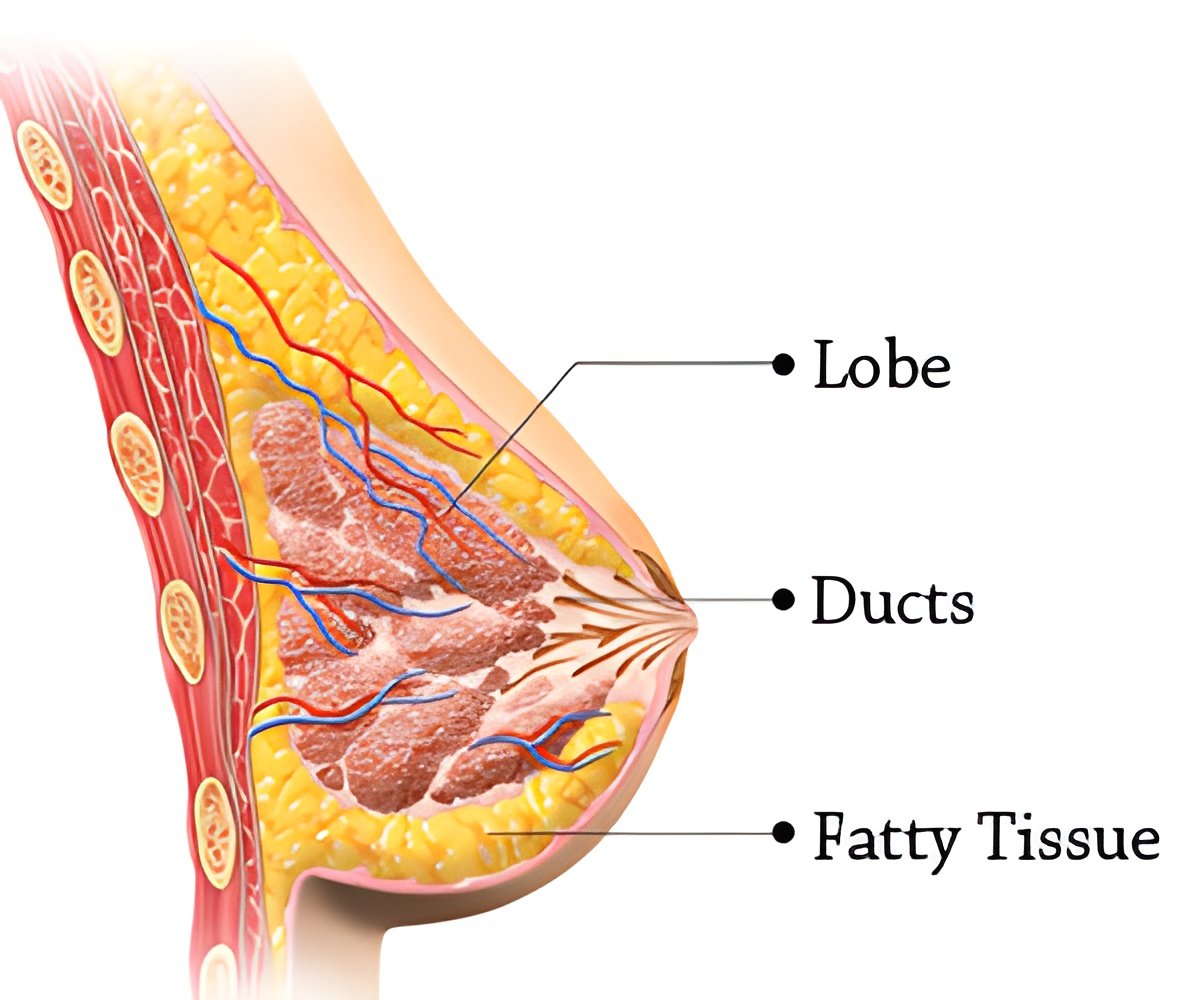
For patients with ductal carcinoma in situ (DCIS) and early-stage invasive breast cancer, APBI has been shown to be an alternative to standard whole-breast irradiation, allowing for a reduction in the duration of radiation therapy. The technique, by reducing the amount of normal healthy breast tissue treated with radiation, may also reduce side effects of treatment and improve cosmetic outcomes. In updating the guidelines, Dr. Shah led a group of physicians - appointed by the American Brachytherapy Society, with expertise in breast cancer and breast brachytherapy - to develop a consensus statement. The new guidelines recommend the technique for a broader group of patient by allowing younger patients to be eligible as well as those with DCIS. The authors' consensus is that the appropriate candidates for APBI include patients aged 45 years or older; all invasive histologies and ductal carcinoma in situ; tumors 3 cm or less; node negative; estrogen receptor positive/negative; no lymphovascular space invasion; and negative margins.
"The updated guidelines support clinicians by offering them the ability to appropriately select patients for APBI, and data that supports the techniques," said Dr. Shah. "Guidelines allow for the selection of patients who can finish radiation treatment in one week or less, compared to the traditional period of three to six weeks, and potentially a reduction in side effects depending on the APBI technique."
The previous APBI guidelines, also led by Dr. Shah, were developed in 2013. Since then, multiple randomized trials have been published which have increased the amount of data available to develop the updated guidelines. The authors' recommendation was based on review of literature including randomized trials, prospective studies, multi-institutional series, and single-institution reports addressing clinical outcomes and toxicities with APBI.
Advertisement