The U.S. Food and Drug Administration (FDA) has approved the blood-thinning drug Effient tablets (prasugrel) to reduce the risk of blood clots from forming in patients who undergo angioplasty, a common procedure to unblock a clogged coronary artery.
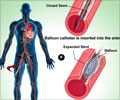
During an angioplasty, a balloon is used to open the artery that has been narrowed by atherosclerotic plaque. Often, a tiny wire mesh scaffold (stent) is inserted into the blood vessel to help keep the artery open after the procedure.Platelets in the blood can clump around the procedure site, causing clots that can lead to heart attack, stroke, and death.
Prasugrel works by inhibiting platelet activation and subsequent aggregation by blocking the P2Y12 adenosine diphosphate (ADP) receptor on the platelet surface. Antiplatelet agents prevent platelets from clumping or sticking together, which can result in clogged arteries and may lead to heart attack or stroke.
Effient was studied in a 13,608-patient trial comparing it to the blood-thinning drug, Plavix (clopidogrel), in patients with a threatened heart attack or an actual heart attack who were about to undergo angioplasty.
The fraction of patients who had subsequent non-fatal heart attacks was reduced from 9.1 percent in patients who received Plavix to 7.0 percent in patients who received Effient. While the numbers of deaths and strokes were similar with both drugs, patients with a history of stroke were more likely to have another stroke while taking Effient. In addition, there was a greater risk of significant, sometimes fatal bleeding seen in patients who took Effient.
"Effient offers physicians an alternative treatment for preventing dangerous blood clots from forming and causing a heart attack or stroke during or after an angioplasty procedure," said John Jenkins, director of the Office of New Drugs, in the FDA's Center for Drug Evaluation and Research. "Physicians must carefully weigh the potential benefits and risks of Effient as they decide which patients should receive the drug."
Advertisement
Effient is manufactured by Eli Lilly and Company of Indianapolis, in partnership with Tokyo-based Daiichi Sankyo Ltd.
Advertisement
Source-Medindia
GPL