Using toxic molecules to develop therapeutic agents is not easy. Researchers are beginning to understand how some molecules control the activities of bacteria.
‘Using toxic molecules to develop therapeutic agents is not easy. Biologists are now beginning to understand how some molecules control the activities of bacteria and will help develop drugs with many different applications.’





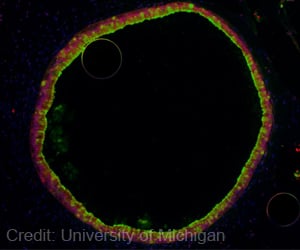
The Luk Research Group is focusing its attention on Pseudomonas aeruginosa, a microbe that causes many diseases and illnesses, including cystic fibrosis. Like other bacteria, P. aeruginosa produces a range of molecules that guides various activities. Among these molecules are rhamnolipids, which are made up of rhamnose sugar rings and fatty acids, and are able to reduce the surface tension of water. While rhamnolipids have been studied for decades, scientists don't really know how they affect bacterial behavior. Enter Luk, who, along with his research team, designs molecules that control such behavior. Their latest creation is a class of molecules called synthetic disaccharide derivatives (DSDs), which take over the chemical signaling of rhamnolipids to control activities such as biofilm formation, bacterial adhesion and swarming motility.
Lead author Nischal Singh G'15 and the rest of the Luk team have also discovered a subset of DSDs that dominates the function of rhamnolipids, and has demonstrated capacity for a range of new, unexpected bioactivities. The latter includes phenotypic switching, in which bacteria abandon their original phenotypes to "change" into two different phenotypes, and bacterial adhesion, considered the first step in colonization and biofilm formation.
"Biologists know how rhamnopilids are made by bacteria, and can knock out their production, but they don't fully understand how rhamnolipids work--specifically, how they control different types of bacterial activities," Luk says.
In addition to being non-toxic and biodegradable, rhamnolipids can withstand extreme temperatures, salinity and acidity. As a result, they have many useful chemical and biological properties.
Advertisement
"By determining the important structural features of DSDs, we've figured out how to control the behavior of P. aeruginosa," Luk adds. "At the same time, DSDs have enabled a series of novel biological phenomena that are starting to reveal how rhamnolipids work--something that has baffled scientists for a long time. It's exciting to be on the brink of discovery." The researchers have published their findings in ChemBioChem (John Wiley & Sons, 2015).
Advertisement