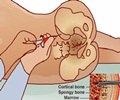
“Our products enable physicians to aspirate bone marrow in their own office with only local anesthesia, which is revolutionary,” said Dr. Wade McKenna, Biologic Therapies’ Chief Medical Director.
The devices are available in a variety of lengths, allowing fast and easy bone marrow draws throughout the skeletal anatomy, including the tibia, humerus and calcaneus bones.
“The ability to aspirate a patient’s marrow, concentrate the marrow in a centrifuge to derive the maximum number of regenerative cells and growth factors, and then give the concentrated cells and growth factors back to the patient in a physician’s office makes regenerative cell therapy a much easier, more available and less costly procedure. The entire point-of-care procedure takes less than 30 minutes. Of course, the Bio-MAC and Bio-CORE are also used in hospitals and surgery centers, said McKenna.
Source-Medindia