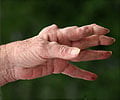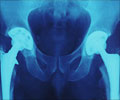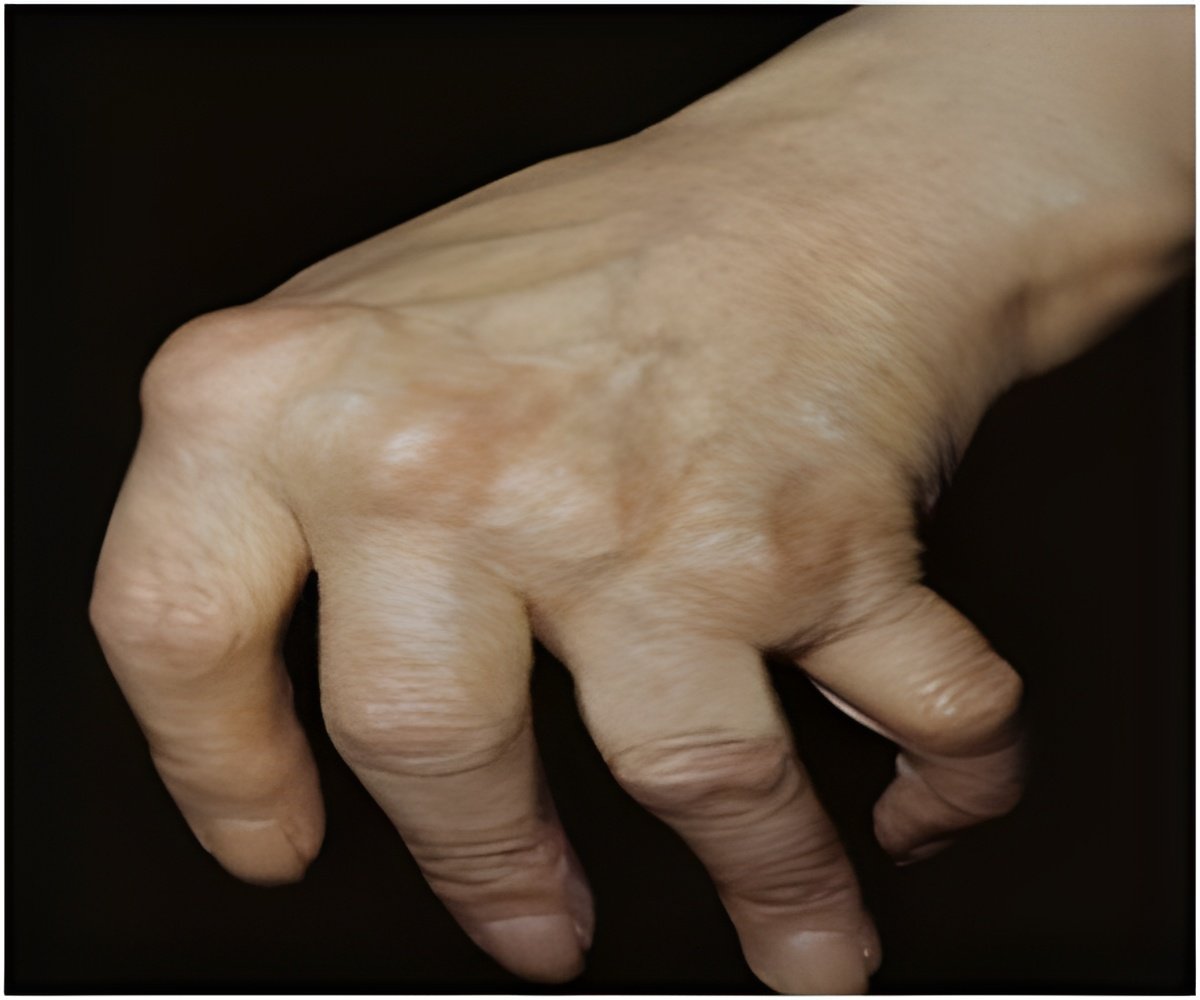
From a randomized clinical trial, Jonas K. Eriksson, M.Sc., of the Karolinska Institutet, Sweden, and colleagues measured monthly sick leave and disability pension days in patients who did not achieve low disease activity after three to four months of methotrexate therapy. The patients were divided into groups to receive additional biological treatment with infliximab or conventional combination treatment with sulfasalazine plus hydroxychloroquine. Of 204 eligible patients, 105 were assigned to biological and 99 to conventional treatment.
The baseline average work loss was 17 days per month in both groups. The average changes in work loss at 21 months were -4.9 days per month in the biological and -6.2 days per month in the conventional treatment group, according to the study results.
"Our analysis showed that early and aggressive treatment in methotrexate-resistant patients not only stops the trend of increasing work loss days, as in patients with mainly established RA, but partly reverses it. However, we did not find any difference between treatment arms, indicating that the significantly improved disease control associated with infliximab treatment over a one-year period and the better radiological results after two years did not translate into less work loss," the study concludes. "The substantially higher cost of infliximab relative to conventional treatment needs to be weighed against the greater incidence of short-term adverse events leading to discontinuation of conventional treatment."
(JAMA Intern Med. Published online July 1, 2013. doi:10.1001/jamainternmed.2013.7801..)
Editor's Note: Authors made conflict of interest disclosures. The study was funded by the Swedish Rheumatism Association and Schering-Plough/Merck Sharp and Dohme. Please see the article for additional information, including other authors, author contributions and affiliations, financial disclosures, funding and support, etc.
Commentary: Not Better but Quite Good
Advertisement
"In the real-world situation of sequential use of combinations, first excluding and only after that including biological agents, the outcome might not match that achieved after simultaneous randomization, but the fine study done by Eriksson and colleagues indicates that it may be good enough," Yelin concludes.
(JAMA Intern Med. Published online July 1, 2013. doi:10.1001/jamainternmed.2013.7812. Available pre-embargo to the media at http://media.jamanetwork.com.)
Advertisement
Source-Newswise