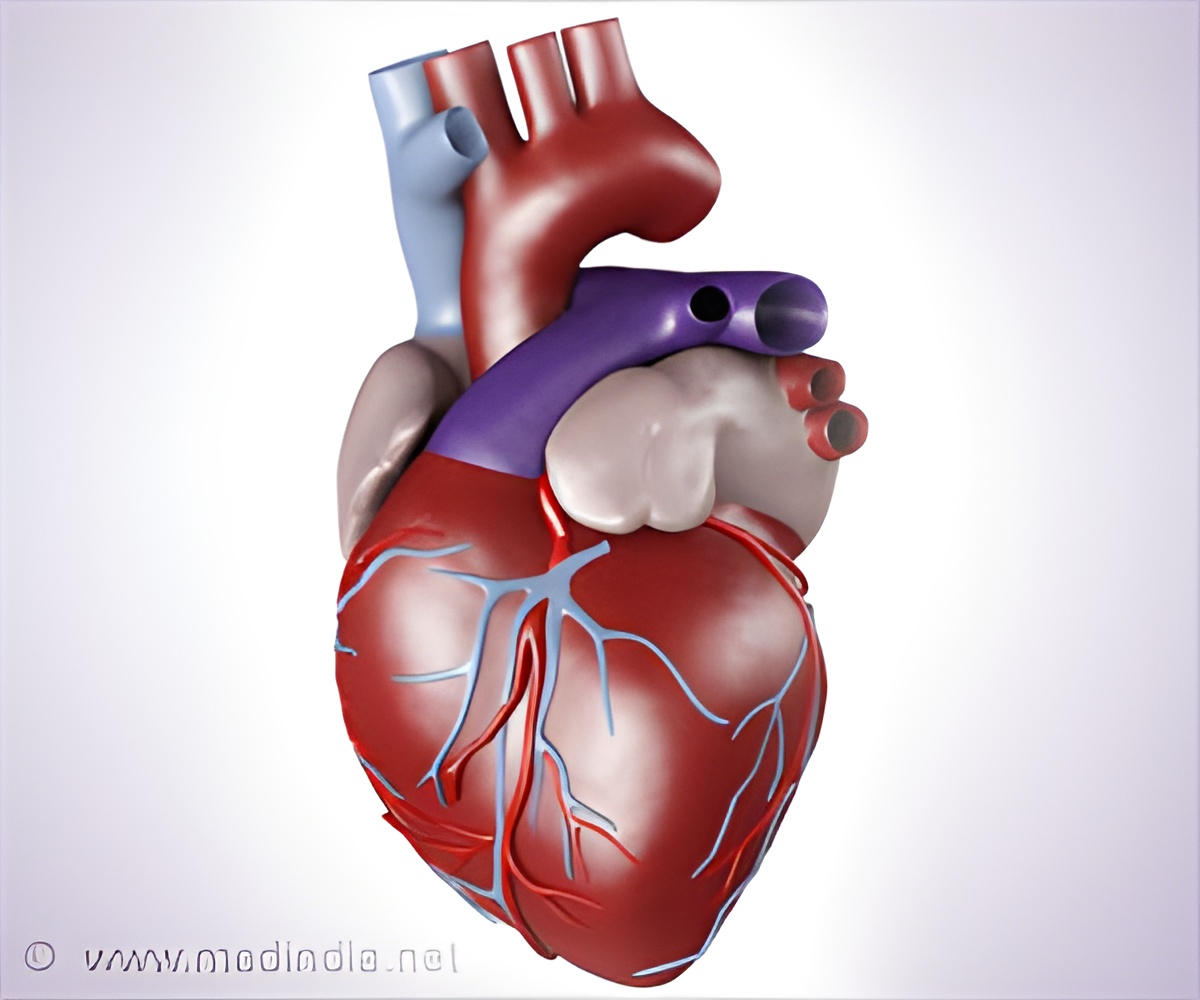
A new scaffold- biodegradable stent- made from cornstarch instead of metal has been successfully implanted in a patient M.P. Singh, 65, for the first time ever in India. This procedure was carried out by Dr. Ashok Seth, chairman, Fortis-Escorts Heart Research Institute. Dr. Seth is also the principal investigator for this trial in India. This biodegradable scaffold stent is manufactured by a US based medical device company, Abbott. It was first fitted in a patient from Australia four years ago.
"Clinical trials are ongoing at across 100 centers all over the world but it is too early to talk about costs and long-term results," said Dr Seth. "Unlike the metal stents, which remain in the body even after they have served their purpose, these biodegradable scaffolds will dissolve after the artery is repaired, turning into carbon dioxide and water after about two years," he said. Once the scaffold dissolves, the arteries contract and relax like normal arteries. Other potential advantages over metal stents are that the patient is able to undergo MRI and even stop blood-thinning medicines without any risk of formation of clots in the arteries. "Another big advantage will be that a patient with multiple stents, who had reached a no-option situation—they need repeat stenting or surgery but cannot go for it because of the long length of metal inside the arteries—will have both options available to them," he said.
The chances of restenosis is 5% i.e. formation of new blockages within three to six months of stenting at the site of angioplasty are same as that with metal stents. Five institutes across India will carry out trials over the next one year. These institutes include- Fortis Escorts (Delhi), Apollo (Chennai), Care Hospital (Hyderabad), Madras Medical Mission and SAL hospital (Ahmedabad). According to Dr. Seth these stents would be available commercially in about one and half year.
Source-Medindia