Although research seeking a plausible AIDS vaccine has been going on for decades, scientists have not made a breakthrough till date. Now, researchers have explained why anti-HIV antibodies are ineffective in blocking the deadly infection.
Researchers from the California Institute of Technology (Caltech) have said that the success has not been achieved in this field partly due to the fact that our body's natural HIV antibodies simply don't have a long enough reach to effectively neutralize the viruses they are meant to target."This study helps to clarify the obstacles that antibodies face in blocking infection, and will hopefully shed more light on why developing an effective vaccine for HIV has proven so elusive," said Pamela Bjorkman, the Max Delbruck Professor of Biology at Caltech.
Usually, Y-shaped antibodies are considered ideal to neutralize viruses-i.e., blocking their entry into cells and preventing infection-when both arms of the Y are able to reach out and bind to their target proteins at more or less the same time.
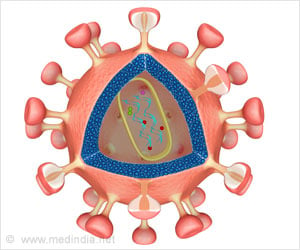
In the case of HIV, antibodies that can block infection target the proteins that stud the surface of the virus, which stick out like spikes from the viral membrane.
However, an antibody can only bind to two spikes at the same time if those spikes fall within its span-the distance the antibody's structure allows it to stretch its two arms.
"When both arms of an antibody are able to bind to a virus at the same time, there can be a hundred- to thousand fold increase in the strength of the interaction, which can sometimes translate into an equally dramatic increase in its ability to neutralize a virus. Having antibodies with two arms is nature's way of ensuring a strong binding interaction," said Joshua Klein, a Caltech graduate student in biochemistry and molecular biophysics and the study's first author.
Advertisement
One, called b12, binds a protein known as gp120, which forms the upper portion of an HIV's protein spike. The other, 4E10, binds to gp41, which is found on a lower portion of the spike known as the stalk.
Advertisement
After looking at the 4E10 antibody, they found that having two arms conferred almost no advantage over having only one arm and that larger versions of the antibody were less effective than smaller ones.
Thus, vaccines designed to elicit antibodies similar to 4E10 might face many obstacles and the same goes for b12 as well.
On looking more closely at their data, the researchers realised that the benefits of having two arms--even for b12--were much smaller than those seen for antibodies against viruses like influenza.
Thus, the researchers concluded that the body's natural anti-HIV antibodies are much less effective at neutralizing HIV than they should be.
Klein explained: "The story really starts to get interesting when we think about what the human immunodeficiency virus actually looks like."
Whereas a single influenza virus's surface is studded with approximately 450 spikes, the similarly sized HIV may have fewer than 15 spikes.
With spikes so few and far between, finding two that both fall within the reach of a b12 or 4E10 antibody--the spans of which generally measure between 12 and 15 nanometers--becomes much more of a challenge.
"HIV may have evolved a way to escape one of the main strategies our immune system uses to defeat infections. Based on these data, it seems that the virus is circumventing the bivalent effect that is so key to the potency of antibodies," said Klein.
The findings have been published in the online early edition of the Proceedings of the National Academy of Sciences (PNAS).
Source-ANI
LIN