A new study has claimed that the world's most popular anti-cholesterol drug Crestor, could be of use in more ways than one. Among patients who currently don't face a high risk of getting a heart disease, Crestor could reduce their risk further by nearly 44 percent.
The results of the study named JUPITER were presented at an annual meeting of the American Heart Association in New Orleans and were published online by the New England Journal of Medicine.The study of 17,802 men and women aged 50 and more demonstrated that 20 milligrams of Crestor taken daily significantly reduced the combined risk of myocardial infarction, stroke, arterial revascularization, hospitalization for unstable angina, or death from cardiovascular causes by 44 percent compared to placebo among men and women with elevated hsCRP but low to normal cholesterol levels.
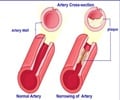
CRP is a protein produced by the liver that plays an important role in inflammatory processes and serves as a biological marker to measure the risk of artery blockage.
The research also showed that for patients in the trial taking Crestor the combined risk of heart attack, stroke or death from heart disease was reduced by 47 percent, the risk of heart attack was cut by more than half, the risk of stroke by nearly half and total mortality was reduced by 20 percent.
"These results provide new information about Crestor's effects on CV risk," said Howard Hutchinson, chief medical officer for pharmaceutical company AstraZeneca, which markets Crestor.
"The JUPITER trial confirmed that Crestor dramatically reduces LDL-C cholesterol levels and has now demonstrated a nearly 50 percent reduction in the risk of heart attack and stroke in a population of patients who had elevated hsCRP but low to normal cholesterol levels," Hutchinson added. "As is appropriate, the medical community, regulators, and guideline committees will now carefully consider these data and any implications for treating patients."
Advertisement
There was no difference between treatment groups for major adverse events, including cancer or myopathy, the report said.
Advertisement
Crestor has now received regulatory approval in over 95 countries. Nearly 15 million patients have been prescribed Crestor worldwide.
Source-AFP
TAN