Pfizer, the world's biggest drug maker, said Tuesday it was terminating development of two medicines aimed at treating pain and anxiety disorders to focus on higher-potential development programs.
It would halt "phase 3 development programs" for investigational compounds for fibromyalgia and for generalized anxiety disorder, the company said in a statement."While confident in the safety of these compounds, we don't believe that they provide significant benefit over other therapies," said Pedro Lichtinger, president of Pfizer's primary care business unit.
The decision to terminate the two programs will enable the business unit "to allocate additional resources to higher-potential development programs as part of its continuing effort to deliver greater value to patients and Pfizer shareholders," the company said in a statement.
Pfizer said it would push ahead for approval for using its Lyrica drug to treat generalized anxiety disorder, a chronic condition with symptoms including persistent anxiety, exaggerated worry and tension.
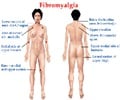
The company would also continue studies for the treatment of fibromyalgia, a condition of chronic widespread pain.
In June 2007, Lyrica became the first FDA-approved treatment for the management of fibromyalgia, and Pfizer said it continued to support research and education in this complex pain condition.
Advertisement
Source-AFP
SRM