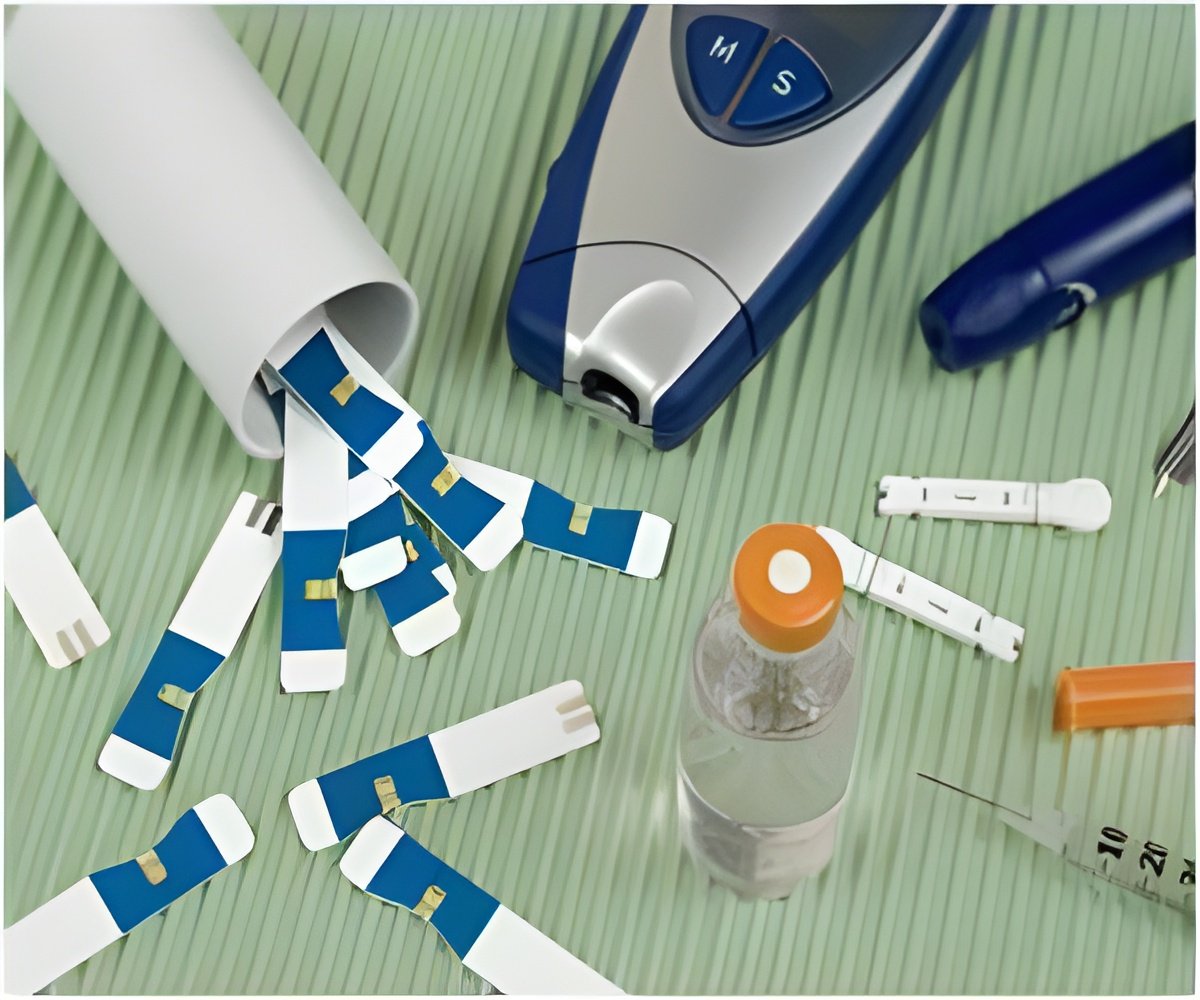
Abbott Laboratories, Washington, announced a recall of about 359 million glucose test strips used for monitoring blood glucose level because the strips may give false low readings which could result in an over or under treatment of diabetes. According to the company spokesman Scott Davies these chemically treated paper strips were manufactured in the United Kingdom between January and May 2011.
These strips may fail to absorb sufficient blood leading to false results. This would happen if the strips are stored in a warm environment for a long time. Low results may lead diabetic patients to raise their blood glucose or they may fail to treat elevated blood glucose due to false, low reading which pose a health threat. Abbott Diabetes Care pulled recalled strips marketed under brand names- Precision Xceed Pro, Precision Xtra, Medisense Optium, Optium, OptiumEZ and RellOn Ultima.
The agency said that patients should note the time it takes to register the result. Results that take more than 5seconds to register should indicate a defective glucose test strip. FDA has asked patients to be cautious about unusually low glucose readings and pay attention to symptoms of hyperglycemia and hypoglycemia. Director of the Office of InVitro Diagnostics, Alberto Gutierrez, PhD, said that the FDA and Abbott are working together to find the source of the defect.
Source-Medindia