The US Food and Drug Administration has warned on Thursday that taking the abnormal heart rhythm drug Multaq may prove fatal.
The drug was approved by the FDA to treat patients with short-term arrhythmia lasting less than six months and the health agency said that over 241,000 Americans may have been prescribed the medicine.
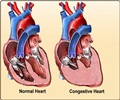
The warning comes following a study that looked into the drug’s effectiveness among patients who have the long-term form of the disease, known as atrial fibrillation.
The FDA revealed that the study found twice as many deaths occurring among patients who took the drug compared to those who did not take it.
Source-Medindia