A cervical cancer vaccine from GlaxoSmithKline has been authorised for sale in the United States, the British drug giant announced.
The US Food and Drug Administration has approved the use of Cervarix in girls and young women aged 10 to 25, it said, three years after rivals Merck and Sanofi-Pasteur launched their vaccine on the US market."The approval of Cervarix will bring an important new cervical cancer vaccine to girls and young women," said Deirdre Connelly, president of North American Pharmaceuticals at GlaxoSmithKline, in a company statement.
GSK added that the vaccine, which is administered in three doses over a maximum of six months, would be available in the United States by the end of the year.
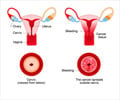
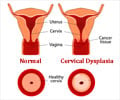
Cervarix protects against two types of the human papillomavirus (HPV) that cause cervical cancer, types 16 and 18.
US drugs giant Merck and Sanofi-Pasteur, the vaccinations unit of European laboratory Sanofi-Aventis, have sold their vaccine Gardasil in the United States since June 2006. It protects against HPV types six, 11, 16 and 18.
Source-AFP
SRM