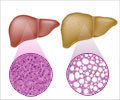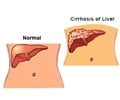
NASH affects 2 to 5 percent of Americans. An additional 10 to 20 percent of Americans have fat in their liver, but no inflammation or liver damage, a condition called “fatty liver.” Although having fat in the liver is not normal, by itself it probably causes little harm or permanent damage. If fat is suspected based on blood test results or scans of the liver, this problem is called nonalcoholic fatty liver disease (NAFLD). If a liver biopsy is performed in this case, it will show that some people have NASH while others have simple fatty liver.
Both NASH and NAFLD are becoming more common, possibly because of the greater number of Americans with obesity. In the past 10 years, the rate of obesity has doubled in adults and tripled in children. Obesity also contributes to diabetes and high blood cholesterol, which can further complicate the health of someone with NASH. Diabetes and high blood cholesterol are also becoming more common among Americans.
Two studies presented at the 75th Annual Scientific Meeting of the American College of Gastroenterology investigated the effetiveness of potential treatments for NASH, one assessing pentoxifylline, used to improve blood flow. It works by decreasing the thickness (viscosity) of blood. The other study was an analysis of pioglitazone, an insulin sensitizer, compared to vitamin E.
Claudia O. Zein, M.D. and colleagues at the Cleveland Veterans Affairs Medical Center and the Cleveland Clinic in Cleveland, OH conducted a double‐blinded, randomized, placebo‐controlled trial of pentoxifylline in patients with NASH.
“The higher frequency of biochemical and histological improvement observed in our study among patients who received pentoxifyllie compared to placebo, suggest both a reason for hope and a need for further studies,” added Dr. Zein.
Advertisement
Although insulin sensitizers have been the main focus for developing new treatments for NASH, the study by Dr. Zein and another study indicates that pathways other than insulin resistance could be used for developing effective treatments for NASH.
Advertisement
Non‐diabetic adult patients with NASH were randomized to receive pioglitazone (a drug used in Type 2 diabetes), vitamin E, or placebo and followed after 96 weeks of treatment.
“Although Adipo‐IR was not different from placebo in either treatment group after 96 weeks, there were trends for improvement with pioglitazone and worsening of Adipo‐IR with vitamin E, suggesting that the positive effect of vitamin E on liver histology is independent of changes in adipose tissue insulin resistnce,” explained Dr. Bell.
The mechanism by which vitamin E improves liver histology remains unknown but it is thought to be due to its intrahepatic antoxidant effects. Dr. Bell’s finding that increases in Adipo‐IR correlated with worsening fibrosis, if confirmed, could be an important non‐invasive test for monitoring liver fibrosis in patients with NASH.
Source-Medindia