Review the side-effects of Midostaurin as documented in medical literature. The term "side effects" refers to unintended effects that can occur as a result of taking the medication. In majority of the instances these side-effects are mild and easily tolerable, however sometimes they can be more severe and can be detrimental.
If the side effects are not tolerable adjusting the dosage or switching to a different medication can help to manage or overcome side effects. If you have any doubts or questions, we recommend seeking advice from your doctor or pharmacist.
Common: Nausea, vomiting, neutropenia (low white blood cell counts), edema (fluid accumulation), fatigue, fever
Gastrointestinal: Inflammation of the mucous membranes, piles, raised pancreatic enzymes, abdominal discomfort or pain, constipation, bleeding
Cardiovascular: Prolonged QT interval (an abnormality in the electrical activity of the heart), fluctuations in blood pressure, heart attack, pericardial effusion (fluid accumulation around the heart), clot formation in a blood vessel
Central nervous system: Headache, dizziness, vertigo, insomnia, difficulty in attention, tremor
Blood: Low blood counts
Respiratory: Nasopharyngitis, sinusitis, bleeding from the nose, difficulty in breathing, lung inflammation, pleural effusion (fluid accumulation around the lung), cough, pain in the oropharynx, upper respiratory tract infection, lung toxicity
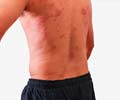
Skin: Skin lesions in the form of petechiae, maculopapular rash, erythema multiforme, and erysipelas increased sweating, dry skin
Others: Swelling of eyelids, herpes virus infection, increased levels of creatinine, joint pain, raised levels of uric acid in blood, muscle pain, high blood glucose levels, device-related infections, increase weight, high blood calcium levels, fever
• Midostaurin may impair fertility in both men and women of childbearing age. It is advised to consider the option of using a sperm or egg bank to store sperms or eggs before starting treatment.
• The use of midostaurin capsules in pediatric patients is not recommended as the safety and effectiveness of the drug has not established in this group.
If the side effects are not tolerable adjusting the dosage or switching to a different medication can help to manage or overcome side effects. If you have any doubts or questions, we recommend seeking advice from your doctor or pharmacist.
Common: Nausea, vomiting, neutropenia (low white blood cell counts), edema (fluid accumulation), fatigue, fever
Gastrointestinal: Inflammation of the mucous membranes, piles, raised pancreatic enzymes, abdominal discomfort or pain, constipation, bleeding
Cardiovascular: Prolonged QT interval (an abnormality in the electrical activity of the heart), fluctuations in blood pressure, heart attack, pericardial effusion (fluid accumulation around the heart), clot formation in a blood vessel
Central nervous system: Headache, dizziness, vertigo, insomnia, difficulty in attention, tremor
Blood: Low blood counts
Respiratory: Nasopharyngitis, sinusitis, bleeding from the nose, difficulty in breathing, lung inflammation, pleural effusion (fluid accumulation around the lung), cough, pain in the oropharynx, upper respiratory tract infection, lung toxicity
Skin: Skin lesions in the form of petechiae, maculopapular rash, erythema multiforme, and erysipelas increased sweating, dry skin
Others: Swelling of eyelids, herpes virus infection, increased levels of creatinine, joint pain, raised levels of uric acid in blood, muscle pain, high blood glucose levels, device-related infections, increase weight, high blood calcium levels, fever
Other Precautions :
• Pregnancy test has to be performed within seven days starting the midostaurin treatment and the drug should be initiated only if the pregnancy test is negative.• Midostaurin may impair fertility in both men and women of childbearing age. It is advised to consider the option of using a sperm or egg bank to store sperms or eggs before starting treatment.
• The use of midostaurin capsules in pediatric patients is not recommended as the safety and effectiveness of the drug has not established in this group.