- Glomerulonephritis - (https://www.ncbi.nlm.nih.gov/books/NBK560644/ )
- Histology, Nephron - (https://www.ncbi.nlm.nih.gov/books/NBK554411/ )
- Immunobiology: The Immune System in Health and Disease - (https://www.ncbi.nlm.nih.gov/books/NBK10752/)
- T Cell-Mediated Immunity - (https://www.ncbi.nlm.nih.gov/books/NBK10762/ )
- Pathophysiology of proteinuria - (https://pubmed.ncbi.nlm.nih.gov/12631062/ )
- Azotemia - (https://pubmed.ncbi.nlm.nih.gov/30844172/ )
- Peripheral Edema - (https://www.ncbi.nlm.nih.gov/books/NBK554452/)
- The practical management of fluid retention in adults with right heart failure due to pulmonary arterial hypertension - (https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6915055/ )
- Nephrotic syndrome: components, connections, and angiopoietin-like 4-related therapeutics - (https://pubmed.ncbi.nlm.nih.gov/24854282/)
- Nephritic Syndrome - (https://www.ncbi.nlm.nih.gov/books/NBK562240/)
- Minimal Change Disease - (https://www.ncbi.nlm.nih.gov/books/NBK560639/ )
- Role of respiratory viruses in exacerbations of primary nephrotic syndrome - (https://pubmed.ncbi.nlm.nih.gov/3005537/ )
- Focal Segmental Glomerulosclerosis - (https://www.ncbi.nlm.nih.gov/books/NBK532272/)
- Successful Reuse of Kidney Graft After Early Recurrence of Primary Focal and Segmental Glomerulosclerosis - (https://pubmed.ncbi.nlm.nih.gov/34118304/)
- Glomerular Filtration Barrier Assembly: An insight - (https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5003421/ )
- A short history of 'glomerulus' - (https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4432456/)
- Treatment outline and risk stratification - (https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3697423/)
- Treatment of idiopathic membranous nephropathy in adults - (https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6768298/ )
- IgA Nephropathy - (https://www.ncbi.nlm.nih.gov/books/NBK538214/)
- Membranoproliferative glomerulonephritis - (https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2887509/ )
- Postinfectious glomerulonephritis - (https://pubmed.ncbi.nlm.nih.gov/22885383/)
- Poststreptococcal Glomerulonephritis - (https://www.ncbi.nlm.nih.gov/books/NBK538255/ )
- Goodpasture Syndrome - (https://www.ncbi.nlm.nih.gov/books/NBK459291/ )
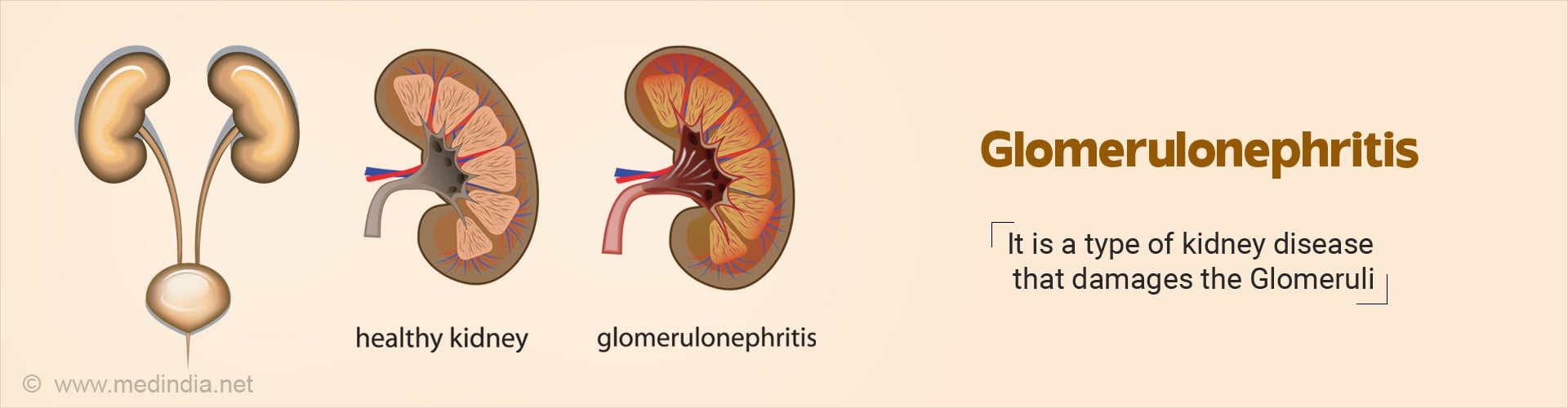
What is Glomerulonephritis?
Glomerulonephritis (glo·mer·u·lo·ne·phri·tis) is a word used to describe a spectrum of kidney diseases that result in damage of glomeruli due to inflammation, infection of the functional units of nephron which filters blood.
When the kidney is damaged, it cannot get rid of wastes and extra fluid in the body. If the illness continues, the kidneys may stop working completely. The damage can cause leakage of protein and sometimes the red cells into the urine (1✔ ✔Trusted Source
Glomerulonephritis
Go to source).
Each nephron contains a structure called the glomerulus which is actually a complex matrix of highly specialized blood vessels through which blood passes and the waste products of the body are purified. The condition is also called glomerular nephritis (2✔ ✔Trusted Source
Histology, Nephron
Go to source).
There are various types of glomerular diseases which are classified based on the type of mechanism of inflammation and section of the glomeruli that are affected. Broadly, the immune mechanism that causes the inflammation is of two types:
I. Antibody-mediated (Humoral) Immunity is the aspect of immunity mediated by the activity of B lymphocytes and circulating antibodies. The cells responsible for antibody-mediated immune response are (3✔ ✔Trusted Source
Immunobiology: The Immune System in Health and Disease
Go to source):
- B cells or lymphocytes are responsible for antibody responses.
- Effector T cells for the cell-mediated immune response which is controlled by regulatory T cells.
Antibody-mediated immune response is as follows (3✔ ✔Trusted Source
Immunobiology: The Immune System in Health and Disease
Go to source):
a) B cells are activated by antigen and helper T cells.
b) Antibodies against the antigen are produced by the plasma cells. These antibodies protect the host in 3 ways namely neutralization, opsonization and complement activation.
In neutralization, the antibodies bind to the pathogens and inhibit the toxic effects of the pathogen. In opsonization by coating the pathogens, antibodies ingest and kill the pathogen by a process known as opsonization. Antibodies trigger activation of the complement system (part of the immune system) which enhances opsonization and kills few bacterial cells.
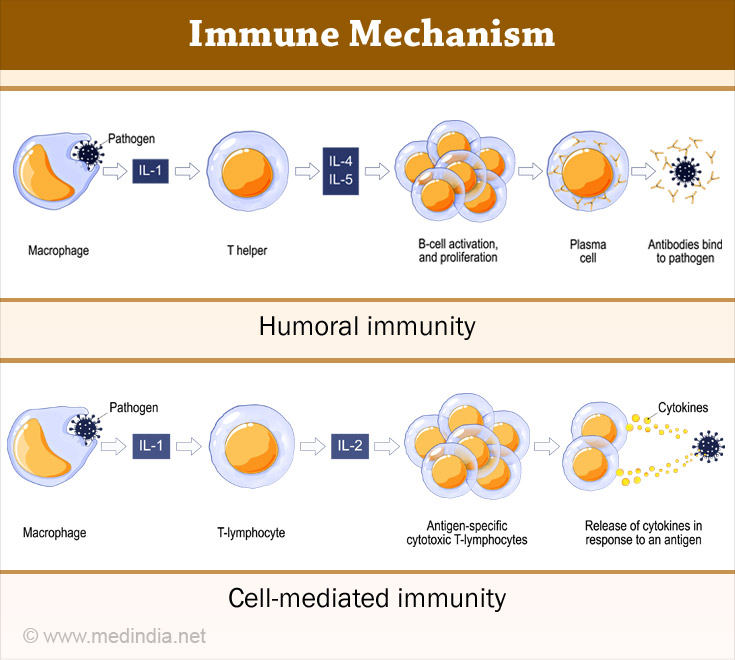
II. Cell-mediated Immunity is an immune response triggered by phagocytes T cells and cytokines released in response to an antigen. T lymphocytes are responsible for cell-mediated immunity. Cell-mediated immunity does not involve antibodies (4✔ ✔Trusted Source
T Cell-Mediated Immunity
Go to source).
Injury to glomeruli results in a variety of signs and symptoms of disease including:
| Proteinurias caused by | Altered permeability of capillary walls (5✔ ✔Trusted Source Pathophysiology of proteinuria Go to source) |
| Hematuria caused by | Loss of capillary wall integrity |
| Azotemia caused by | Impaired filtration and build-up of nitrogenous wastes (6✔ ✔Trusted Source Azotemia Go to source) |
| Oliguria or Anuria caused by | Reduced glomerular filtration |
| Edema caused by | Loss of intravascular oncotic pressure (7✔ ✔Trusted Source Peripheral Edema Go to source) |
| Hypertension caused by | Fluid retention & altered renal regulation of blood pressure (8✔ ✔Trusted Source The practical management of fluid retention in adults with right heart failure due to pulmonary arterial hypertension Go to source) |
Syndromes Related to Glomerulonephritis
Several syndromes related to glomerulonephritis have been described.
a) Nephrotic Syndrome: Proteinuria in excess of 3.5 grams in 24 hours, accompanied by (9✔ ✔Trusted Source
Nephrotic syndrome: components, connections, and angiopoietin-like 4-related therapeutics
Go to source):
- Proteinuria - Passage of large molecular weight proteins like albumin and other tubular proteins.
- Edema or swelling of the body due to fluid retention.
- Hypoalbuminemia or low albumin levels.
- Hyperlipidemia or high lipids (fats).
- Lipiduria (passing of fat in the urine)
- Loss of surface tension
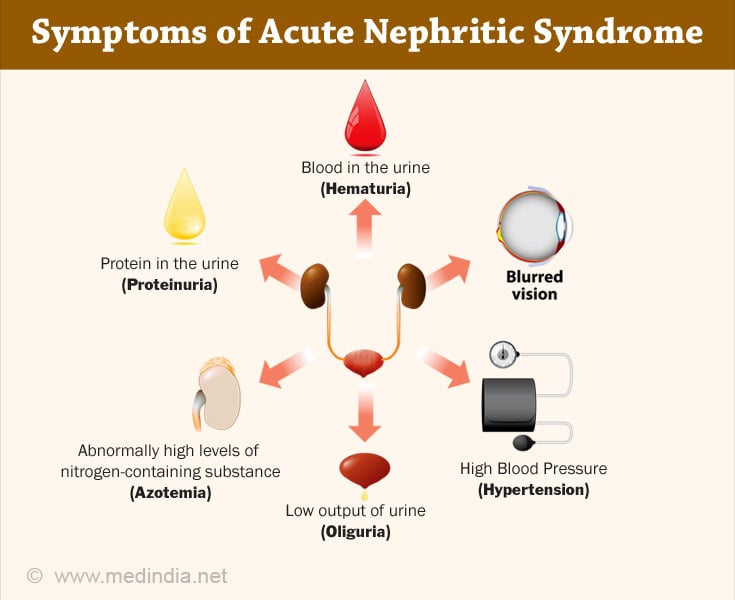
b) Acute Nephritis or Nephritic Syndrome:
The clinical manifestation of Nephritic Syndrome are (10✔ ✔Trusted Source
Nephritic Syndrome
Go to source)
- Hematuria – traces of blood in the urine or blood stained urine
- Variable proteinuria – protein in the urine
- Azotemia – increase of urea
- Hypertension – increase of blood pressure
Types of Glomerulonephritis
Classfication of Glomerulonephritis based on clinical presentation are as follows (1✔ ✔Trusted Source
Glomerulonephritis
Go to source):
1. Non – proliferative glomerulonephritis
- Minimal Change Glomerulonephritis
- Focal Segmental Glomerulosclerosis
- Membranous Glomerulonephritis
- Anti-Glomerular Basement Membrane Disease or Goodpasture’s syndrome
2. Proliferative glomerulonephritis
- Antibody-Mediated IgA Nephropathy
- Membranoproliferative Glomerulonephritis
- Infection related Glomerulonephritis
- Rapidly Progressive Glomerulonephritis
A description of the various types of glomerulonephritis is provided in brief.
Rapidly Progressive Glomerulonephritis
Results in hematuria, oliguria and acute renal failure.
Although much overlap exists, the various types of glomerular diseases may be classified by syndrome which manifests and provides the predominant clinical picture:
| Nephrotic Syndrome | Nephritic Syndrome | |
| Minimal change disease | +++++ | - |
| Membranous glomerulonephritis | ++++ | + |
| Focal segmental glomerulosclerosis | ++++ | + |
| IgA nephropathy | +++ | ++ |
| Membranoproliferative GN | ++ | +++ |
| Acute post-infectious GN | + | ++++ |
Minimal Change Glomerulonephritis
Minimal Change Disease - is the most common cause of the nephrotic syndrome in childhood. In a prospective study of untreated children with the nephrotic syndrome, minimal change disease was found in 76.6%.
In contrast, minimal change disease accounts for only 10% to 30% of adult cases of nephrotic syndrome. In both children and adults, these percentages vary in different parts of the world (11✔ ✔Trusted Source
Minimal Change Disease
Go to source).
The clinical onset of nephrotic syndrome may be associated with an upper respiratory infection or with routine prophylactic immunizations (12✔ ✔Trusted Source
Role of respiratory viruses in exacerbations of primary nephrotic syndrome
Go to source). Other genetic and environmental factors may also be important.
Clinical Course: Most patients with minimal change disease develop mild edema around the eyes (periorbital) as the initial complaint (11✔ ✔Trusted Source
Minimal Change Disease
Go to source).
The proteinuria in minimal change disease is said to be "selective", that is, it is composed primarily of albumin.
Microscopic hematuria or traces of blood in the urine is rare with reported frequencies of 13 to 36%.
Hypertension is also unusual in minimal change disease.
Treatment - Majority have spontaneous resolution after the inciting agent is withdrawn. 90% are steroid responsive cases in less than 10 % and kidney failure from minimal change disease is rare, but relapses are common.
Focal Segmental Glomerulosclerosis (FSGS)
Focal segmental glomerulosclerosis is the most common cause of nephrotic syndrome in adults (12✔ ✔Trusted Source
Role of respiratory viruses in exacerbations of primary nephrotic syndrome
Go to source). It also comprises 10% of childhood disease.
Patients usually present with -
- Proteinuria.
- Sometimes with microscopic hematuria.
- Hypertension is also common.
- Many variants of FSGS are elucidated with varying response to steroid , immunusupressive medications.
Humans with idiopathic focal segmental glomerulosclerosis initially have a high glomerular filtration rate and evidence of hyperfiltration, suggesting that hyperfiltration and increased intercapillary glomerular pressure may be mediators of this disease.
A similar pattern of segmental sclerosis may be seen in a number of distinct glomerular diseases with different pathogenetic mechanisms and clinical courses (e.g. IgA nephropathy).
Clinical Course - Focal segmental glomerulosclerosis may be primary (idiopathic) or secondary to a number of etiologic agents including (13✔ ✔Trusted Source
Focal Segmental Glomerulosclerosis
Go to source):
- Unilateral renal agenesis – born with only a single kidney
- Renal ablation – removal of kidney
- Sickle cell disease
- Morbid obesity (with or without sleep apnea)
- Congenital cyanotic heart disease
- Heroin nephropathy
- HIV nephropathy
- Aging kidney
Steroid therapy may induce remission in 20-40% of children and fewer adults.
Adults may progress to renal failure rapidly over 2-3 years (15-20%) or more slowly over 10-12 years.
People of African descent tend to have worse outcomes.
Focal segmental glomerulosclerosis commonly recurs following transplantation (50-80%) (14✔ ✔Trusted Source
Successful Reuse of Kidney Graft After Early Recurrence of Primary Focal and Segmental Glomerulosclerosis
Go to source).
Membranous Glomerulonephritis
Membranous glomerulonephritis is an antibody-mediated disease. To understand the mechanism of damage requires some understanding of the glomerulus in detail.
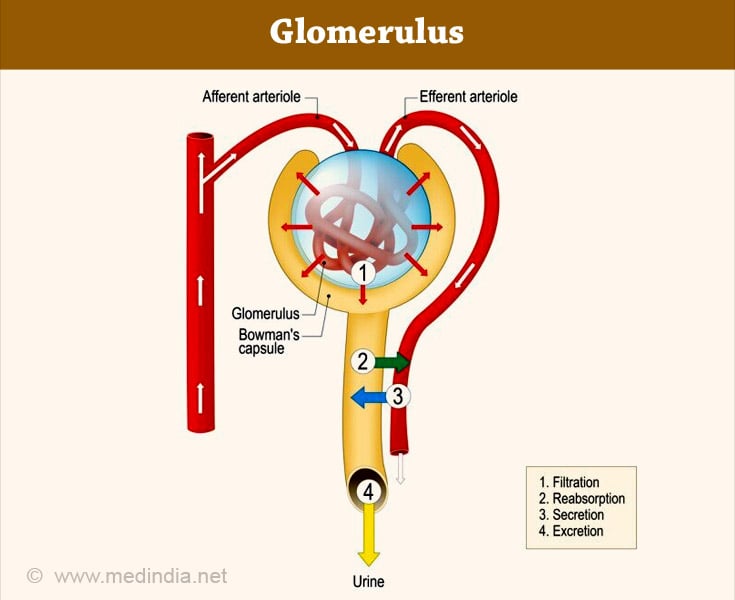
The glomerulus is a cluster of capillaries involved in the filtration of the blood to form urine. The glomerulus is located within the Bowman’s capsule within the kidney (15✔ ✔Trusted Source
Glomerular Filtration Barrier Assembly: An insight
Go to source). The glomerulus was so named by the Italian anatomist Marcello Malpighi (16✔ ✔Trusted Source
A short history of 'glomerulus'
Go to source). Glomerulus receives blood from afferent arteriole and drains into efferent circulation. A glomerulus and a Bowman's capsule constitute a renal corpuscle, the initial blood-filtering component of a nephron. Glomerular filtration rate (GFR) is a test to check the function of the kidneys. The test estimates the rate at which blood is filtered through all of the glomeruli.
The immune complexes localize to the subepithelial aspect of the capillary loop. That is, between the outer aspect of the basement membrane and the podocyte (epithelial cell).
The immune complexes develop in situ or, less likely, by the deposition of circulating immune complexes of antibody and antigen.
Sometimes the antibody may bind to the glomerulus that acts like antigen or to a foreign antigen implanted on the capillary wall.
Approximately, 25 to 30% of cases are secondary. Common associations include:
- Systemic lupus erythematosus and other connective tissue disorders
- Drugs (gold, penicillamine, non-steroidal anti-inflammatory agents)
- Hepatitis B, syphilis, quartan malaria, leprosy, schistosomiasis
- Carcinoma, melanoma, leukemia, non-Hodgkin's lymphomas
Membranous glomerulonephritis is more common in adults and most patients are older than 30 years at diagnosis (17✔ ✔Trusted Source
Treatment outline and risk stratification
Go to source).
Membranous glomerulonephritis accounts for 35-50% of cases of the adult nephrotic syndrome.
Most patients present with (17✔ ✔Trusted Source
Treatment outline and risk stratification
Go to source)-
- Heavy proteinuria - most commonly in the nephrotic range that is insidious in onset.
- A few patients have accompanying microscopic hematuria.
Clinical Course - The course of untreated idiopathic membranous glomerulonephritis is variable. Of patients presenting with the nephrotic syndrome and a normal serum creatinine:
- 30% will have a spontaneous complete remission and a stable GFR for up to 20 years.
- 25% will have a spontaneous partial remission with a stable GFR (Glomerular Filtration Rate).
- 20-25% experience persistent nephrotic syndrome with stable or very slowly progressive loss of GFR.
- 20 to 25% of patients progress to end-stage renal failure over a 20 to 30-year follow-up.
Patients in whom a causative agent is identified, usually respond to treatment of the underlying disorder, or withdrawal of the offending agent.
Patients with idiopathic membranous require treatment with a course of steroids with cyclophosphamide for a period of at least 6 months (18✔ ✔Trusted Source
Treatment of idiopathic membranous nephropathy in adults
Go to source).
Antibody Mediated IgA Nephropathy
IgA nephropathy, first described in 1968, is the most common form of primary glomerulonephritis in the world.
It is an antibody-mediated glomerular disease in which the immune deposits localize to the mesangium. It is not certain whether the deposits form in situ or from circulating immune complexes (19✔ ✔Trusted Source
IgA Nephropathy
Go to source).
Patients with IgA nephropathy usually present with one of three syndromes:
- Macroscopic hematuria or blood in the urine which means the urine is red along with an upper respiratory infection called syn-pharyngitic hematuria.
- Asymptomatic microscopic hematuria (blood cannot be seen with the naked eye, but only under a microscope) seenand variable proteinuria.
- Henoch-Schonlein purpura is the systemic form of the disease process causing IgA nephropathy and occurs more frequently in children than adults (19✔ ✔Trusted Source
IgA Nephropathy
Go to source).
Patients with Henoch -Schonlein purpura manifest skin, joint and intestinal involvement.
A less common presentation is with the nephrotic syndrome - These patients may have advanced disease or normal renal function. In the latter case, the light microscopic features are of minimal change disease but with intense mesangial staining for IgA.
Clinical Course - Renal function progressively worsens in approximately 40% of patients, about half of whom reach end-stage renal failure after 20 years of clinically apparent disease.
Nearly 30% of patients exhibit a benign course with chronic microscopic hematuria, a normal serum creatinine and proteinuria usually less than 1 gram/day.
Hypertension is not uncommon and malignant hypertension develops in about 5% of patients.
Secondary deposits of IgA may occur in chronic liver disease, dermatitis herpetiformis, psoriasis, ankylosing spondylitis, inflammatory bowel disease, carcinoma, celiac disease, IgA monoclonal gammopathy, HIV infection and mycosis fungoides.
Membranoproliferative Glomerulonephritis
Membranoproliferative, or mesangiocapillary, glomerulonephritis (MPGN) is a chronic progressive glomerulonephritis that occurs in older children and adults (20✔ ✔Trusted Source
Membranoproliferative glomerulonephritis
Go to source).
Circulating immune complexes have been identified in 50% of patients and activation of the complement system with hypocomplementemia is a hallmark of MPGN.
The complement system is a part of the immune system that complements the ability of antibodies and the larger white cells to clear any foreign body or pathogens. Over 30 proteins and protein fragments make up the complement system, including serum proteins, serosal proteins, and cell membrane receptors. Sometimes the inflammation caused by antigen and antibody works against the body and the cascade of inflammation produced damages the tissues.
Idiopathic MPGN has several distinct histopathologic and immunopathologic features and three variants have been described.
- Type I MPGN characterized by mesangial and subendothelial immune deposits (20✔ ✔Trusted Source
Membranoproliferative glomerulonephritis
Go to source). - Type II MPGN (dense deposit disease) characterized by interrupted linear deposits in the lamina densa of the basement membrane (20✔ ✔Trusted Source
Membranoproliferative glomerulonephritis
Go to source). - Type III MPGN, an uncommon variant, characterized by features of both type I MPGN and membranous GN, or by disruption of the glomerular basement membrane with an accumulation of new basement membrane material arranged in layers (20✔ ✔Trusted Source
Membranoproliferative glomerulonephritis
Go to source).
Clinical Course - Patients with MPGN may present with the nephrotic syndrome or an abnormal urinary sediment with non-nephrotic proteinuria or with acute nephritis. The diagnosis is suspected when serum complement levels are depressed.
Type I MPGN:
- Is the most frequent primary MPGN.
- Is also often associated with systemic disease, infection and neoplasms.
- Membranoproliferative glomerulonephritis secondary to cryoglobulinemia is strongly associated with Hepatitis C virus infection (20✔ ✔Trusted Source
Membranoproliferative glomerulonephritis
Go to source). - The nephrotic syndrome is the most common presenting illness.
- Type I MPGN is a slowly progressive disease; in 30-40% of patients, the disease remains stable despite persistent nephrotic range proteinuria. Median survival, free of renal failure in both children and adults ranges from 9 to 12 years.
In type II MPGN:
- Acute nephritis and recurrent macroscopic hematuria are the most common presentations.
- Most patients with type II MPGN are younger than 20 years of age, have more persistent C3 depression and more commonly present with nephritis.
- Approximately, 20% of patients remain stable for many years and median survival, free of renal failure ranges from 5 to 10 years.
Infection Related Glomerulonephritis
Post-Infectious Glomerulonephritis - Infectious agents are the most common inciting antigens associated with immune complex- mediated glomerulonephritis (21✔ ✔Trusted Source
Postinfectious glomerulonephritis
Go to source).
Post-Sreptococcal Glomerulonephritis is the most common form of glomerulonephritis in children and occurs following a skin or throat or pharyngeal infection with Group A beta-hemolytic streptococci (a type of bacteria) (22✔ ✔Trusted Source
Poststreptococcal Glomerulonephritis
Go to source).
Post-Infectious Glomeruonephritis has also been associated with other bacterial, viral, parasitic, rickettsial and fungal infections.
The precise nature of the antigens involved in the formation of the immune complexes that deposit in the nephrons (nephritogenic) is unknown.
Streptococcal antigenic substances have been inconsistently detected in glomeruli and circulating immune complexes have been detected in some patients. Since streptococcal antigens do not always cause disease, other mechanisms may be involved, including alterations in IgG or glomerular components making them through activation of alternate complement pathway (22✔ ✔Trusted Source
Poststreptococcal Glomerulonephritis
Go to source).
Antigens derived from infectious agents may bind to glomerular structures and induce development of in situ immune complexes.
Clinical Course - Post-streptococcal glomerulonephritis is primarily a disease of children, 6 to 7 years of age, although it may also be seen in adults and has a different clinical course in them.
The onset is usually abrupt, with a latent period of 7 to 21 days between infection and the development of nephritis. During epidemics, the clinical attack rate is 10-12%, but subclinical disease occurs four times more frequently than an overt disease. Asymptomatic contacts may have hematuria (22✔ ✔Trusted Source
Poststreptococcal Glomerulonephritis
Go to source).
Common initial clinical manifestations of post-streptococcal glomerulonephritis are (22✔ ✔Trusted Source
Poststreptococcal Glomerulonephritis
Go to source):
- Hematuria (micro or macroscopic) - blood in urine
- Edema or swelling of the body
- Oliguria or less urine output
- Hypertension
The acute clinical episode of post-streptococcal glomerulonephritis is usually self-limited and complement levels return to normal within 6 weeks. In most patients, hematuria disappears by 6 months but proteinuria may persist for two years in a third of patients.
Early antimicrobial therapy in affected individuals and family members may prevent the spread of streptococcal infections. Treatment of established infection does not prevent the development of post-streptococcal glomerulonephritis, but may lessen its severity.
The prognosis for complete recovery is excellent in children, even in patients with the nephrotic syndrome or crescentic disease at presentation (22✔ ✔Trusted Source
Poststreptococcal Glomerulonephritis
Go to source).
The prognosis in adults is less favorable, especially when accompanied by initial severe impairment in renal function, persistent proteinuria and the nephrotic syndrome. The development of crescents is more common in adults.
Anti-Glomerular Basement Membrane Disease (GBM)
Anti GBM diasease is separate entity and if involving the lung basement membrane is called GOODPASTURE SYNDROME. It involves formation of antibody against the self basement membrane antigens.
Up to one-third of patients with rapidly progressive glomerulonephritis and anti-GBM antibodies, do not have detectable evidence of pulmonary involvement (anti-glomerular basement membrane disease).
Since the antibody response is directed toward a normal antigen present in the GBM, Goodpasture's syndrome and anti-GBM disease are classic examples of autoimmune disorders.
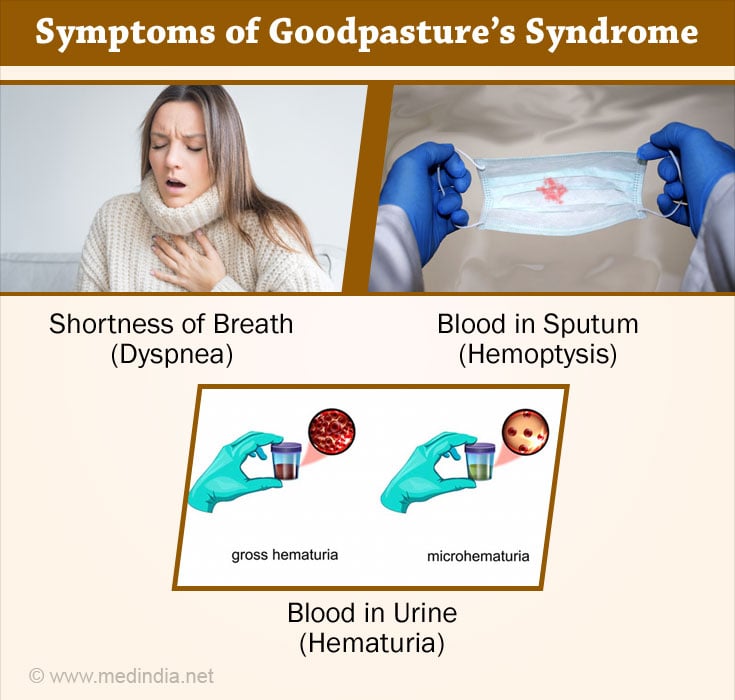
Clinical Course - Goodpasture's syndrome may occur at any time, but peaks in the spring and summer months are common. It frequently begins with a flu-like illness with evidence of pulmonary compromise.
- Progressive dyspnea or breathing difficulty
- Hemoptysis and occasionally ventilatory failure occur
- Hematuria with red blood cell casts
- Variable proteinuria
Treatment - Patients with Goodpasture's syndrome require emergent institution of plasma exchange and immunosuppressive therapy with steroids and cyclophosphamide (23✔ ✔Trusted Source
Goodpasture Syndrome
Go to source).
Prompt institution of therapy usually results in significant improvement in renal function. Patients with an initial serum creatinine less than 3 mg/dl and fewer than 30% crescents have a greater likelihood of responding favorably to therapy.