- Hepatic encephalopathy - (https://en.wikipedia.org/wiki/Hepatic_encephalopathy)
- Know About Hepatic Encephalopathy - (http://he123.liverfoundation.org/diagnosis/what-are-the-stages-of-he/)
- What Are the Stages of Hepatic Encephalopathy? - (https://rarediseases.org/rare-diseases/hepatic-encephalopathy/)
- Volume 2014 (2014), Article ID 236268 - (http://dx.doi.org/10.1155/2014/236268 Bleibel W, Al-Osaimi AMS. Hepatic Encephalopathy. Saudi J Gastroenterol. 2012 Sep-Oct; 185: 301–309. )
- Cordoba J. Hepatic Encephalopathy: From the Pathogenesis to the New Treatments. ISRN Hepatology - (https://rarediseases.org/rare-diseases/hepatic-encephalopathy/)
- García-Martínez R1, Simón-Talero M, Córdoba J. Prognostic assessment in patients with hepatic encephalopathy. Dis Markers. 2011;31(3):171-9 - (doi: 10.3233/DMA-2011-0840)
- Bustamante J, Rimola A, Ventura P, Navasa M, Cirera I, Reggiardo V, Rodés J. Prognostic significance of hepatic encephalopathy in patients with cirrhosis. 1999; 30 (5): 890–895 - (http://dx.doi.org/10.1155/2014/236268 Bleibel W, Al-Osaimi AMS. Hepatic Encephalopathy. Saudi J Gastroenterol. 2012 Sep-Oct; 185: 301–309. )
What is Hepatic Encephalopathy?
Hepatic encephalopathy (HE) refers to the impairment in the brain function that occurs in certain patients with liver disease.
Its severity ranges from a mild condition with no externally apparent abnormalities to a severe form that leads ultimately to serious, life-threatening complications. In the terminal stage, it is also referred to as hepatic coma or coma hepaticum.
Symptoms are related to progressive dysfunction of the brain and may include
- Personality changes
- Impairment of intellectual abilities
- Problems with memory
- Loss of consciousness (coma)
What are the Possible Factors That Play a Role In Hepatic Encephalopathy?
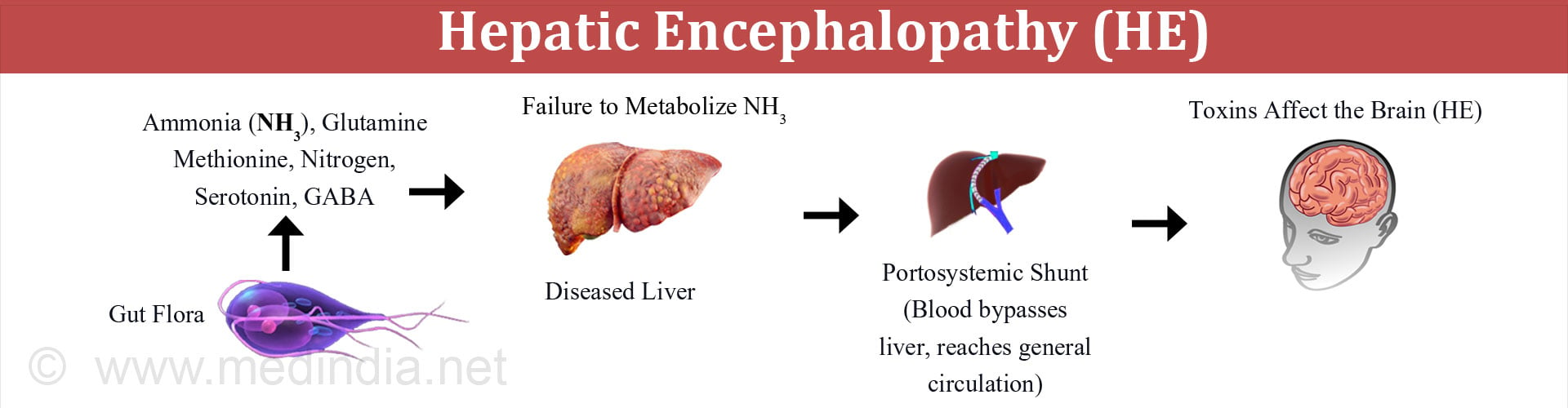
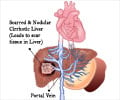
The exact way in which hepatic encephalopathy occurs is poorly understood. An important factor in its development is the entry of portal blood into the systemic circulation through portosystemic shunts. Normally blood from the digestive tract first goes to the liver via the portal vein so that any toxins can be removed. In the presence of a portosystemic shunt, the blood bypasses the liver and reaches the general circulation without getting detoxified. Thus, the toxins in the blood can affect the brain resulting in encephalopathy.
Two possible factors that have been suggested to play a role include:
1. Gut derived neurotoxins such as ammonia
- Under physiological conditions, ammonia enters the portal blood from the digestive tract. It is mainly derived from the colonic bacteria and additionally from deamination (removal of amino group) of the amino acid glutamine in the small intestine.
- When the function of liver is intact, the liver is able to clear most of the ammonia entering the portal venous blood, converting it into urea and glutamine, and thus preventing the entry of ammonia into the systemic circulation.
- However, in the presence of liver damage, the ammonia is shunted into the systemic circulation and reaches the brain, thereby affecting it.
2. Alterations in astrocyte structure and function
- The astrocytes are supporting cells found in the brain. They protect and nourish the neurons or nerve cells and protect them from the harmful effects of ammonia by converting it to glutamine.
- In liver failure, the astrocytes become overwhelmed with the huge quantities of ammonia entering the brain and are unable to metabolize it. There is resultant astrocyte swelling and edema of the brain, which can result in some of the neurological symptoms of hepatic encephalopathy.
Other Possible Factors
- Other effects of increased ammonia levels in brain include impaired function of both excitatory and inhibitory neurotransmitters with overall inhibitory effect. There is a disturbance in the ratio of branched chain amino acids to aromatic amino acids (BCAA/AAA) with enhanced brain uptake of AAA leading to disturbance in neurotransmission.
- Many toxic chemicals formed by the colonic bacteria have been shown to enhance the toxicity of ammonia. These include phenols, short chain fatty acids, mercaptans and oxindole.
- Additional factors that might contribute to elevated blood ammonia levels include impaired kidney function affecting the excretion of ammonia, and muscle wasting which is commonly found in persons with cirrhosis; muscle plays an important role in extrahepatic ammonia elimination.
- Sepsis and inflammation in a previously stable cirrhosis patient has been found to precipitate hepatic encephalopathy (HE). The suggested mechanism is the effect of inflammatory mediators such as cytokines and tumor necrosis factor (TNF) in increasing the brain uptake of ammonia by increasing blood vessel permeability.
What are the Causes of Hepatic Encephalopathy?
Hepatic encephalopathy (HE) can be precipitated by a variety of factors in an otherwise stable cirrhotic patient. These include:
- Excess nitrogen burden – Intake of large amounts of protein, bleeding from esophageal varices, kidney failure with impaired excretion of nitrogenous waste
- Fluid and electrolyte imbalance – Dehydration, hyponatremia (low blood sodium levels), hypokalemia (low blood potassium levels), alkalosis (decreased acid level), hypoxia (lack of oxygen)
- Medications – Benzodiazepines, alcohol, narcotics, antipsychotics
- Infections – Pneumonia, spontaneous bacterial peritonitis, urinary tract infections
- Constipation – Constipation enhances intestinal ammonia production
- Idiopathic – In about a quarter of patients, no precipitating cause can be found

What are the Types of Hepatic Encephalopathy?
Hepatic encephalopathy is sub-classified into three types based on the status of the liver.
- Type A: Associated with acute liver failure.
- Type B: Occurs when there is no primary liver damage and encephalopathy occurs due to portosystemic shunting of blood.
- Type C: Encephalopathy associated with chronic liver disease such as cirrhosis and portal hypertension. The encephalopathy can either be episodic (acute) or persistent (chronic). In some cases, the encephalopathy is subclinical, and the term ‘minimal’ encephalopathy is used to describe such cases.
What are the Stages of Hepatic Encephalopathy?
HE is assessed according to the severity of the symptoms. The most commonly used grading system is the West Haven grading system, which is as follows:
- Grade 0 (Minimal HE or MHE) – Difficult to detect clinically; subtle impairment in memory, concentration and intellectual functions. Slight impairment of coordination that may manifest as poor work performance or ability to drive (incidences of traffic violations while driving). If such occurrences are brought to the physician’s notice, the patient may be referred for neuropsychiatric evaluation and followed up regularly to monitor the condition. At present, there are no drugs to treat minimal HE.
- Grade 1 (Mild HE) – Presence of mood changes, depression, irritability, a decreased attention span and sleep issues
- Grade 2 (Moderate HE) -Associated with increasing forgetfulness, slurred speech, inappropriate behavior, inability to do simple mental tasks like basic math, shaking of hands and writing difficulties
- Grade 3 (Severe HE) –Characterized by marked sleepiness, disoriented in space and time, extreme anxiety and strange behaviour
- Grade 4 (Coma) – Patient loses consciousness and passes into a comatose state
What are the Symptoms of Hepatic Encephalopathy?
The clinical features of hepatic encephalopathy can present in acute, chronic or fulminant forms.
Acute HE – Acute hepatic encephalopathy may occur in the setting of chronic liver disease due to some precipitating factor; it may also occur suddenly in the absence of previous liver disease.
- It is characterized by development of an acute confusional state, neuromuscular abnormalities resulting in problems maintaining posture, and increased rate of breathing.
- Patients usually recover when the precipitating factor is removed or with improvement of liver function.
Chronic HE – Chronic hepatic encephalopathy can occur as:
- Recurrent episodes of encephalopathy which may be spontaneous, due to aggravating factors or discontinuation of treatment. It is characterized by abrupt onset and resolution of symptoms. Symptoms are similar to acute HE described above.
- Persistent symptoms of encephalopathy resistant to treatment.
Features of chronic persistent encephalopathy include confusion and loss of orientation, presence of involuntary abnormal movements such as tremor, increased muscle tone, difficulty in speech, poor attention span, and reduced mental alertness and motor activity.
Fulminant HE – The symptoms begin as an acute encephalopathy and patient gradually slips into a coma.
How is Hepatic Encephalopathy Diagnosed?
Diagnosis of hepatic encephalopathy is one of exclusion. Other diseases can also present with similar neurological and psychiatric manifestations.
Points suggesting a diagnosis of hepatic encephalopathy include:
- Presence of neuropsychiatric manifestations in persons with liver dysfunction
- Lab tests indicative of liver dysfunction such as elevated serum bilirubin, markedly increased liver enzymes, decreased albumin levels and presence of clotting abnormalities

- Electrolyte disturbances such as hyponatremia and hypokalemia
- Elevated serum ammonia levels
- Excluding other causes of encephalopathy by lab studies and radiological imaging such as CT scan and MRI.
- Electroencephalogram (EEG) shows high amplitude, low frequency waves, though this is non-specific to HE. It may be helpful in the presence of cirrhosis with neurological abnormalities. EEG is not necessary to make a diagnosis of HE.
- Neuropsychiatric tests:
- The number connection test (NCT). This test is most often employed at the bedside. It involves circles containing the numbers from 1 to 13 and the letters from A to L; the patient is asked to match the number to the letter such as 1-A, 2-B and 3-C. The result is based on the time taken including time taken to correct errors.
- Line drawing test – This test assesses motor speed and accuracy. The person is required to draw a line within a labyrinth route provided. The borders should not be touched or crossed. The time taken and the number of errors is determined.
- Circle dotting test – The patient is asked to place a dot in each of the 100 circles provided. This is a test of motor speed.
- Psychometric Hepatic Encephalopathy Score (PHES) – Five paper pencil tests were combined to form the PHES to assess attention, memory (to a lesser extent), visual perception, visuo-spatial orientation, motor speed and accuracy, visual construction, and concentration.
- The Block Design Test (BDT test) – This test assesses speed and accuracy. A set of coloured blocks is given to the patient to arrange in a pattern shown on a card.
- The Digital Symbol Test (DST test) – The patient is given a set of 9 boxes divided into an upper and lower half. A number is given on the upper part and the patient has to fill the lower box with a symbol relevant to the number. Results are given by the number of boxes filled correctly in 90 seconds.
- Critical flicker frequency test – This test is used to assess presence of hepatic retinopathy in the setting of HE, since retinal glial cells also play a role in the detoxification of ammonia to glutamine.
An interesting observation of the application of the neuropsychiatric tests was that cirrhosis patients with apparently normal mental status fared badly in these tests (MHE). These patients displayed marked improvement following liver transplant.
How is Hepatic Encephalopathy Treated?
The goal of medical treatment in hepatic encephalopathy is fourfold, namely
- General and supportive care
- Identification and elimination of the aggravating factors
- Reduction of the nitrogenous load from the gut
- Measures to enhance ammonia clearance
General and Supportive Measures
- General measures include frequent bedside monitoring of the patient’s mental status.
- If the patient is comatose, admission to the intensive care unit and endotracheal intubation may be necessary.
- Protein intake should be restricted initially and appropriate nutrition duly administered to the patient either by mouth or nasogastric feeding depending on the condition of the patient.
Identifying Precipitating Factors and their Elimination
As mentioned earlier, several factors may precipitate an attack of HE. A careful history and examination needs to be undertaken to establish the presence of such factors and promptly remove them.
- Bleeding from the digestive tract should be ruled out by stool analysis and the introduction of a nasogastric tube.
- Constipation which can increase ammonia production should be ruled out.
- The presence of infections should be assessed by culture of blood, urine, ascitic fluid and/or pleural fluid as needed.
- Drug abuse should be detected through urinary screening for benzodiazepines, other sedatives and narcotics.
- The presence of fluid and electrolyte disturbances such as alkalosis, hypokalemia, hyponatremia and dehydration possibly due to diuretic effect should be determined.
- The presence of abnormal collateral circulation must be looked for such as splenorenal shunt when no other precipitating factor is found.
Decreasing the Nitrogenous Burden in the Gut
The following measures may be instituted to reduce the nitrogenous load in the gut.
1. Lactulose and lactilol – These are disaccharide sugars that are not absorbed in the gut, and are metabolized by intestinal bacteria to lactic acid and organic acids.
The gut lumen becomes acidified by metabolism of lactulose by the colonic bacteria and this in turn promotes conversion of ammonium to ammonia and passage of ammonia from the tissues into the gut lumen. Acidification of the gut decreases the amounts of ammonia generating coliform bacteria, while at the same time increasing the amounts of non-ammonia generating lactobacilli. Additionally, lactulose functions as a cathartic reducing bacterial load in the colon.
Care must be taken to avoid overdosing since it can lead to severe diarrhea, reduced blood volume and electrolyte disturbances that could precipitate HE.
2. Antibiotics
Rifaximin, a derivative of rifampicin is a non-absorbable antibiotic increasingly used to treat acute exacerbations of HE. It is better tolerated and safer than neomycin, which was the choice earlier.
Measures to Enhance Ammonia Clearance
1. Use of Prebiotics, Probiotics and Synbiotics
Prebiotics are naturally occurring substances found in whole grains, vegetables and fruits. They promote the growth and activity of colonic bacteria. Probiotics are microorganisms that supplement the intestinal bacterial flora. Synbiotics are combinations of prebiotics and probiotics.

2. Zinc therapy
The role of zinc has not been established in the treatment of HE and further research is necessary. Deficiency of zinc has been shown to cause disturbance in levels of neurotransmitters such as GABA and norepinephrine.
Zinc might increase the levels of ornithine transcarbamoylase, a key enzyme in the urea cycle, which favours conversion of ammonia to urea and subsequent excretion in the urine.
3. L-ornithine l-aspartate (LOLA)
LOLA is a combination of 2 amino acids ornithine and aspartate, which stimulate the urea cycle and increase ammonia elimination from the body. These amino acids increase the levels of glutamate, which is converted to glutamine by utilizing ammonia, a reaction that is catalyzed by the enzyme glutamine synthase.
4. Sodium benzoate, sodium phenylbutyrate, sodium phenylacetate
Sodium benzoate reacts with glycine forming hippuric acid, whose subsequent excretion leads to loss of ammonia.
Sodium phenylbutyrate is converted to phenylacetate, which in turn, combines with glutamine forming phenylacetylglutamine. Phenylacetylglutamine is then excreted by the kidneys with loss of ammonia.
Other agents
The role of other agents such as albumin and branched amino aminoacids (BCAA) is being investigated in HE and their role needs to be established conclusively.
Surgical Treatment
When medical measures fail to yield results, the definitive form of management includes orthotopic liver transplant to reverse liver dysfunction and the symptoms of hepatic encephalopathy.
What is the Diet in Hepatic Encephalopathy?
Diet and nutrition is critical to the management of hepatic encephalopathy.
Vegetable protein versus animal protein
A diet rich in vegetable protein, and excluding meat has been shown to be beneficial in patient with HE.

Optimal quantities of protein intake
The practice of protein restriction has been shown to cause malnutrition with adverse consequences. The recommended amount of protein is approximately 1 g of dietary protein per kilogram body weight per day.
Role of Branched chain aminoacids (BCAA’s)
Branched chain aminoacids may be useful supplements to preserve skeletal muscle mass, with minimal risk of precipitating HE. BCAAs have also been shown to reduce the level of false neurotransmitters, which might contribute to symptoms of HE.
What is the Prognosis of Hepatic Encephalopathy?
In general, hepatic encephalopathy is a dreaded complication of liver failure, and the prognosis remains grave. The cause of hepatic encephalopathy and the underlying state of the liver could impact the outcome though.
- Hepatic encephalopathy associated with acute liver failure carries a poor prognosis, though potentially reversible.
- In cirrhotic patients, the prognosis depends on the aggravating or precipitating factor, though the overall survival rate is low. Studies have suggested that cirrhosis patients having an episode of HE should be candidates for liver transplant. The selection is based on the functional status of the liver, determined by the Model for End-stage Liver Disease (MELD) index.
- Recurrent episodes of HE is seen in patients with huge portosystemic shunts, progressive decline in liver function and hyponatremia (low blood sodium levels).
- Acute on chronic liver failure is the most severe form of decompensated liver function and survival is dictated by other co-morbidities and associated organ failures.
- Hepatic encephalopathy associated with Wilson’s disease or mushroom poisoning calls for an immediate liver transplant.