Manchester-based medical device maker Zilico revealed that it has received the CE Mark approval for its cervical cancer diagnostic device, ZedScan.
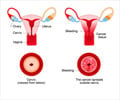
The company had been carrying out trials to test the effectiveness of the device, initially known as APX, over the past few years and said that ZedScan is able to accurately diagnose cervical cancer based on the differences in electrical resistance between normal, pre-cancerous, and cancerous tissue. Apart from being able to accurately diagnose the presence of cancer, a recent independent health economics report has found the device to be cost-effective for healthcare systems, the company said.
“ZedScan has the potential to make a significant impact on how women are managed within the cervical cancer pathway by providing better information to the clinician in real-time and its European approval is an important advancement for clinicians, healthcare systems and patients”, Zilico CEO, Sameer Kothari said.
Source-Medindia