"TAT is widely used in Korea and Japan because we experienced the benefit of cilostazol in terms of major adverse cardiovascular events (MACE) after angioplasty," said Hyo-Soo Kim, MD, PhD, director of cardiac catheterization and coronary intervention at Seoul National University Hospital, Republic of Korea, and the study's principal investigator. "In the numerous clinical studies about angioplasty, we have an impression that the MACE rate is lower in Korean studies than in Western ones."
Although previous studies support the addition of cilostazol to conventional dual anti-platelet therapy, cilostazol is not familiar to many Western physicians because it was developed by a Japanese company that has neither marketed it widely outside Asia nor sponsored a large clinical trial of the drug. "Most clinical evidence supporting cilostazol's benefit comes from investigator-initiated trials like ours," Dr. Kim said. "HOST-ASSURE is the first large-scale randomized trial to directly compare the two treatment strategies and confirm the non-inferiority of TAT compared with DDAT."
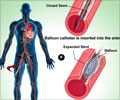
The 2 x 2 factorial trial was designed to compare the two anti-platelet regimens and two types of drug-releasing stents (data to be reported in the future). Patients were randomly assigned to TAT (1,879 patients) or to DDAT (1,876 patients). All patients received 300−600 mg of clopidogrel plus 300 mg of aspirin before angioplasty with (TAT) or without (DDAT) a loading dose of 200 mg of cilostazol. In the TAT group, 100 mg of cilostazol twice daily was added to DAT for a month after the procedure; in the DDAT group, the maintenance regimen was 150 mg of clopidogrel with aspirin.
The primary endpoint was the occurrence of events including cardiovascular-related death, non-fatal heart attack, stroke and major bleeding at one month after angioplasty. That total was 23 patients (1.2 percent) in the TAT group and 27 patients (1.4 percent) in the DDAT group, demonstrating non-inferiority of the three-drug regimen compared with the double-dose two-drug treatment. The TAT group had fewer heart attacks (one patient vs. five). The DDAT group had fewer side effects (eight patients), but none of the 34 patients in the TAT group who had side effects developed life-threatening or severe adverse reactions. The most frequent side effects of cilostazol are headache and gastrointestinal trouble, and most of the side effects are reversible, Dr. Kim noted. Patients will be followed for three years.
"This study provides evidence for the already popular adjunctive use of cilostazol in clinical practice in Asia – in particular in Korea and Japan," Dr. Kim said. "If TAT is equivalent to a potent regimen such as DDAT that is used for high-risk patients, TAT would be preferred because it has additional vascular biologic benefit on top of its anti-platelet effect."
Advertisement