‘This will help in the development of cell treatments for regenerative medicine and better management of vitamin A resistant conditions.’
Tweet it Now
The study by researchers at the Babraham Institute and their international collaborators is published today in Proceedings of the National Academy of Sciences (PNAS).For regenerative medicine, the aim is to be able to generate a cell that can be directed to become any other cell, such as brain cells, heart cells and lung cells. Cells with this ability are present in the early embryo (embryonic stem cells, ESC) and give rise to the many different cell types in the body.
For the purposes of regenerative medicine,adult cells from a patient should be forced to regress back to possessing embryonic-like capabilities and to 'forget' their previous identity.
A cell's identity is established at the DNA level by epigenetic changes to the DNA. These changes don't alter the order of the DNA letters but control which parts of the genome can be read and accessed.
Consequently, every different cell type has a unique epigenetic fingerprint, enforcing and maintaining specific patterns of gene expression appropriate to the cell type. To reverse cells back to the naïve pluripotent state this epigenetic layer of information has to be lost to open up the full genome again.
Advertisement
They looked in particular at the epigenetic modification where a methyl chemical tag is added to the C letters in the DNA sequence. Embryonic stem cells show low levels of this C tagging, called cytosine methylation, but in established cell types much more of the genome is marked by this modification. Removing the methyl tags from the DNA, called demethylation, is a central part of achieving pluripotency and wiping epigenetic memory.
Advertisement
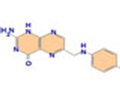
They found that vitamin A enhances epigenetic memory erasure in naïve ESC by increasing the amount of TET enzymes in the cell, meaning greater removal of methyl tags from the C letters of the DNA sequence. In contrast, they found that vitamin C boosted the activity of the TET enzymes by regenerating a co-factor required for effective action.
Dr Ferdinand von Meyenn, postdoctoral researcher at the Babraham Institute and co-first author on the paper, explained "Both vitamins A and C act individually to promote demethylation, enhancing the erasure of epigenetic memory required for cell reprogramming." Dr Tim Hore, previously a Human Frontier Long Term Research Fellow at the Babraham Institute, now Lecturer at the University of Otago, New Zealand and co-first author on the paper, continued: "We found out that the mechanisms of how vitamins A and C enhance demethylation are different, yet synergistic."
The improved understanding of the effect of vitamin A on the TET2 enzyme also potentially explains why a proportion of patients with acute promyelocytic leukemia (once considered the deadliest form of acute leukemia) are resistant to effective combination treatment with vitamin A. By providing a possible explanation for this insensitivity for further investigation, this work could point the way to better management of the vitamin A resistant cases.
Professor Wolf Reik, Head of the Epigenetics Programme at the Babraham Institute, said "This research provides an important understanding in order to progress the development of cell treatments for regenerative medicine. It also enhances our understanding of how intrinsic and extrinsic signals shape the epigenome; knowledge that could provide valuable insight into human disease, such as acute promyelocytic leukemia and other cancers. Putting the full picture together will allow us to understand the full complexity of the epigenetic control of the genome."
Source-Medindia