A supplemental new drug application (sNDA) for Valtrex (valacyclovir HCl) caplets has been approved by the U.S. Food and Drug Administration (FDA) for the treatment of cold sores in healthy adults. This was announced by GlaxoSmithKline which also added that this new application for Valtrex makes it the first one-day, oral antiviral medication proven to shorten the duration of a cold sore outbreak.
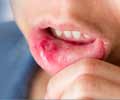
Cold sores, also known as herpes labialis and commonly referred to as fever blisters, are ulcers or blisters on the lip and outer edges of the mouth caused by the herpes simplex virus. People who develop cold sores are advised to exercise high levels of caution, like washing hands immediately after touching the sore, as they are highly contagious, like other herpes infections. Though, it is estimated that that 20 to 40 per cent of adults develop cold sores at some point in time, it is not clear as to why some people get cold sores while others do not. Several factors like stress, exposure to sun, a cold, fever or flu could trigger the herpes simplex virus, which starts reproducing almost immediately, causing damage to skin cells that could lead to characteristic blisters.Valtrex, is now, the only drug which offers the convenience of a one-day treatment for cold sores. It is also the only drug which offers the convenience of once-daily dosing for the suppression of genital herpes outbreaks, as well as a three-day treatment for recurrent outbreaks of genital herpes. Treatment with Valtrex is most effective when it is initiated at the first instance of a cold sore and should be administered for more than a day. The efficacy of using Valtrex for treatment after the development of clinical signs of a cold sore has, however, not yet been established.