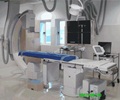
For many Americans living with a heart transplant, invasive heart-muscle biopsies that check for organ rejection are a fact of life.
Now, a simple blood test that analyzes a patient’s genes has been evaluated by leading transplant centers and shows that it can accurately detect the absence of heart transplant rejection, according to new data reported in an invited editorial authored by a consensus team of international heart transplant experts, including a physician-scientist at NewYork-Presbyterian Hospital and Columbia University Medical Center, and available today in the online edition of the Journal of Heart and Lung Transplantation (JHLT).In 2006, the CARGO (Cardiac Allograft Rejection Gene Expression Observational Study) study reported the utility of a gene expression profiling (GEP) test, called AlloMap® molecular expression test, which led to the test’s commercial availability in January 2005. Currently 40 transplant centers in the U.S. offer the test.
'GEP testing is not only less invasive and less risky than biopsy, it also monitors the absence of organ rejection and raises suspicion of damage before any damage to the heart happens. Biopsy records damage that has already occurred,' says Dr. Mario Deng, the article’s senior author. He is director of cardiac transplantation research and associate professor of clinical medicine at Columbia University College of Physicians and Surgeons, and cardiologist at NewYork-Presbyterian/Columbia.
The editorial’s first author is Dr. Randall C. Starling, vice chairman of cardiovascular medicine and section head of heart failure and cardiac transplant medicine at Cleveland Clinic.
Approximately 30 percent of all heart transplant patients reject their new heart at least once in the first year after transplantation. When testing reveals organ rejection, a patient’s immunosuppressive regimen is adjusted.
Based on new data, in more than 99 percent of cases, the AlloMap test successfully predicted heart-muscle biopsies that showed absence of moderate or severe acute cellular organ-transplant rejection. These results confirmed the findings of the CARGO study.
Advertisement
'Additional clinical roles for this new mode of transplantation rejection monitoring will be identified in ongoing studies by our international study group,' adds Dr. Deng.
Advertisement
The heart-muscle biopsy has for decades been the most reliable method available for detecting rejection of the transplanted heart. Invasive heart biopsies are performed initially once a week for the first two months, then every four-to-eight weeks for the first year, and then finally tapered to once every three to six months, often for the patient’s lifetime.
Currently, the AlloMap test is available to heart transplant patients, ages 15 and older, after two months post-transplantation.
Source-Newswise
SRM