- Apalutamide is a drug under development for the treatment of prostate cancer.
- It extends the period of metastasis-free survival (the time of progression from local to other organ or lymph nodes).
- It may be used to delay the progress of nonmetastatic, castration-resistant prostate cancer.
Senior author Eric Small, MD, deputy director of the Helen Diller Family Comprehensive Cancer Center at UCSF, who presented the data at the ASCO-GU Symposium, says, "This trial's results suggest that the availability of apalutamide should offer men with nonmetastatic, castration-resistant prostate cancer a treatment that can delay or prevent the development of metastases and other complications associated with disease progression."
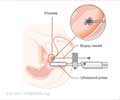
Androgen-deprivation therapy, either through surgical removal of the testicles or the use of drugs that suppress testosterone production, is standard treatment for men with metastatic prostate cancer and is also used for nonmetastatic cancer. Unfortunately, androgen deprivation stops working for almost all patients, leading to what is called castration-resistant disease. In such patients whose cancer has not yet spread, a rapid rise in prostate-specific antigen (PSA) levels warns of the near-term development of metastases, the major cause of complications and death from prostate cancer.
How Apalutamide works in prostate cancer
Apalutamide binds to the androgen receptor, blocking its activation by testosterone and other androgens. Apalutamide is being developed by The Janssen Pharmaceutical Companies of Johnson & Johnson, which sponsored this study. A previous phase 2 clinical trial of apalutamide for men with nonmetastatic, castration-resistant prostate cancer at high risk of progression showed that the drug was well tolerated and achieved responses in most patients.
The current trial was conducted at 322 sites in 26 countries in North American, Europe and the Asia-Pacific. More than 1,200 patients enrolled in the trial between October 2013 and December 2016. All participants had nonmetastatic prostate cancer that had stopped responding to androgen-deprivation therapy and a rapid PSA doubling time, indicating an elevated risk for metastasis. Participants were randomized to receive a daily oral dose of either apalutamide or a placebo and were evaluated every 16 weeks for signs of disease progression.
Incidence of Prostate Cancer
The incidence of the cancer in men goes up with their age and in men who are over the age of 80 years, it is almost 80%. Prostate cancer incidence has been going up due to improving longevity.
- It is estimated that approximately 1 in 7 men will be diagnosed with prostate cancer during their lifetime, however only 1 in 33 will die of this disease.
- Overall prostate cancer is the seventh most common cause of death in the United States.
- About 6 in 10 cases are diagnosed in men aged 65 or older,
- The average age at the time of diagnosis is about 66.
- The number of deaths was 20.1 per 100,000 men per year.
- Matthew R. Smith, Fred Saad et al. Apalutamide Treatment and Metastasis-free Survival in Prostate Cancer, The New England Journal of Medicine DOI: 10.1056/NEJMoa1715546