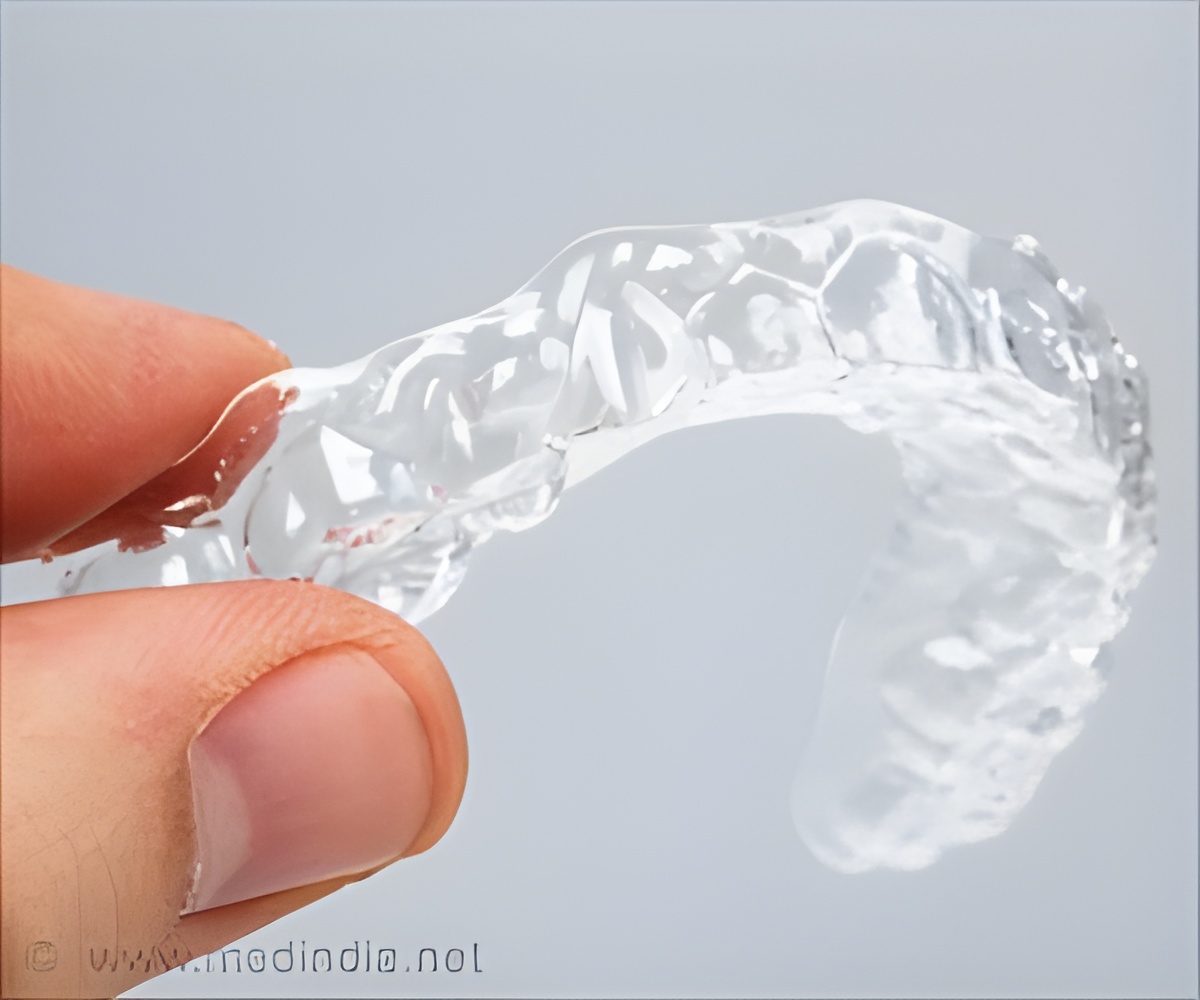
SomnoDent is worn during sleep to provide Continuous Open Airway Therapy by moving the jaw slightly forward. The movement tightens the soft tissue and muscles of the upper airway and prevents apneas during sleep.
The tiny recorder attached to the device measures the hours worn and head position when a patient is wearing the device. The data is transmitted from the micro-recorder to a HIPAA secure cloud portal for remote access by physicians.
“Our patients subjectively report that they use their SomnoDent devices at very high rates of compliance, but this is a way for physicians and dentists to reliably make sure that we are actually reaching those levels of compliance, because we know that the more compliant the patient is, the more effective the treatment.” said Dr. Jagdeep Bijwadia, SomnoMed’s Chief Medical Officer.
Source-Medindia